Cost of inappropriate use of intravenous N-acetylcysteine for acetaminophen toxicity
Introduction
Acetaminophen (APAP) is a commonly prescribed over-the-counter analgesic and antipyretic. It is safe for children and adults if used at recommended doses, but an acute or chronic overdose of APAP can lead to fatal hepatic injury. APAP toxicity is the most common cause of hepatotoxicity-induced acute liver failure from medication overdose in the United States (1). APAP-related overdoses led to 33,520 annual hospitalizations and 78,414 emergency room visits in the United States in 2011 (2). N-acetylcysteine (NAC) can prevent hepatic injury from acute or chronic APAP toxicity by replenishing hepatic storage of glutathione, an enzyme necessary for proper metabolism of APAP to non-toxic metabolites that may then be excreted (3).
Intravenous (IV) NAC is typically a three-dose, 21-hour regimen, however, some protocols suggest a benefit to prolong administration beyond this duration if a patient’s liver enzymes or serum APAP concentration remains elevated (4). A new emerging system in hospital pharmacies is the move to a single bag protocol providing a 150 mg/kg bolus and 12.5 mg/kg/hour infusion for a total of 400 mg/kg. This treatment preparation is preferential to the three-bag, 300 mg/kg dosing regimen, as it has shown to be associated with decreased risk of medication dosing errors (5). For APAP toxicity, appropriate NAC therapy initiation depends on the patient's history, clinical presentation, and the type of ingestion (acute vs. chronic). The Rumack-Matthew nomogram can be utilized within 4–24 hours following single, acute ingestion (6), while chronic ingestion severity is determined by elevated liver enzymes and APAP concentration in collaboration with toxicology specialists or regional Poison Control recommendations (7).
The standard duration of therapy for acute APAP toxicity with NAC is widely accepted as the “20–21-hour regimen” (8). Multiple studies attempted to alter this regimen in order to decrease cost and hospital length of stay (4,9). However, to date, the 21-hour regimen is still considered the recommended method of treatment. The National Poison Control Center encourages providers to check hepatic transaminases and serum APAP concentration two hours before the end of therapy. Continuation of therapy is recommended until serum APAP is no longer detectable and alanine aminotransferase (ALT) elevations are trending down, sometimes ranging between 48 and 72 hours of extended therapy (10-12).
The treatment regimen for APAP toxicity with either oral or IV NAC is expensive, as it entails hospital admission, frequent laboratory tests, and medication cost. For that reason, it is crucial to initiate treatment only if medically indicated, according to the widely accepted guidelines. This study's primary objective is to analyze the potential healthcare cost savings when providers follow the recommended treatment regimen for initiating IV NAC treatment for APAP toxicity. The secondary objectives were assessment of missed cost-saving opportunities resulting from non-compliance with treatment regimen recommendations for maximum doses and the length of treatment.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/jhmhp-20-87).
Methods
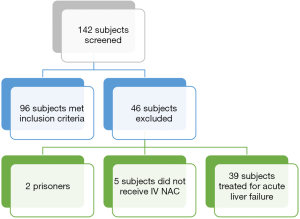
This study is a retrospective, single-center chart review approved by the University of Missouri Institutional Review Board (IRB approval # 2006537). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Given the nature of this study, the institution review board/ethics committee did not require HIPAA Authorization, Assent, and Parental Permission under Exempted criterion. Subjects were identified for inclusion using a dispensing report generated for subjects who received inpatient IV NAC during the 4-year study. Predetermined inclusion criteria included subjects from all ages and genders who received at least one dose of IV NAC for the treatment of APAP toxicity. Predetermined exclusion criteria involved subjects who received IV NAC for liver failure not associated with APAP overdose. Additionally, we excluded pregnant women and prisoners. Receiving one oral NAC loading dose, either at an outside facility or our institution, was not considered an exclusion criterion.
This study included descriptive cost analysis which was completed by the hospital billing department and utilized true cost charges. Total cost of hospitalization was calculated and total medication charges were evaluated separately using the hospital’s group purchasing organization (GPO) pricing. To determine the appropriateness of initiating IV NAC treatment for acute, known time ingestion, we evaluated adherence to the Rumack-Matthew Nomogram. Initiation of IV NAC treatment for acute APAP toxicity was considered inappropriate if it was initiated less than 4 hours following the ingestion, or initiated when the level was below the treatment line on the Rumack-Matthew Nomogram. For those subjects, the entire hospitalization cost was considered a missed cost saving opportunity if no other co-ingestion was documented. However, only medication cost was considered a missed cost saving opportunity if a co-ingestion was present.
Subjects who presented later than 24 hours after ingestion or had a staggered ingestion were categorized as chronic ingestion, and appropriate initiation was based on hepatic transaminases and serum APAP concentration. If the time of ingestion was unknown, then serum APAP concentrations drawn four hours after admission were assessed. For patients with chronic ingestion, only medication cost was considered a missed cost saving opportunity if the initiation of therapy was appropriate, but the therapy exceeded maximum recommended doses or the recommended three infusion cycles. Cost of hospitalization was not considered a missed cost saving opportunity for patients with chronic APAP toxicity, as those patients had to be hospitalized and treated with NAC as per our hospital protocols. Our hospital does not admit medically stable psychiatric patients, even if the intention of ingestion was self-harm, therefore, these subjects’ hospitalization cost was considered inappropriate in the analysis if the admission was not medically warranted.
Laboratory values considered appropriate to discontinue treatment were an undetected level of serum APAP and two measurements of hepatic transaminases trending down. Per manufacturer recommendations, the maximum doses of IV NAC are 15 grams, 5 grams and 10 grams, respectively, for first, second, and third dose. Additional information collected included agents of co-ingestion, adverse events related to NAC, need for subject transfer to a liver transplant center for fulminant liver failure, and death from liver failure during the hospitalization. Baseline characteristics, rate of appropriate initiation based on time of toxicity, percentage of subjects receiving more than three doses, and dosing in the obese population were analyzed with descriptive statistics.
Results
One hundred and forty-two subjects were screened for inclusion based on a dispensing report of all inpatient IV NAC orders administered at our institution in 4 years. A total of 96 adult and pediatric subjects met inclusion criteria and were included in the final analysis (Figure 1). Sixty (68.2%) adult and n=8 (100%) pediatric subjects were females. The average subject ingested at least two other agents in addition to APAP. The most common agents of co-ingestion and other subject characteristics are summarized in Table 1. Of the included, n=56 (58.3%) subjects had acute toxicity with known ingestion time, while n=40 (41.7%) had either chronic or unknown time of ingestion (Table 2). No data elements were missing from the medical records.
Table 1
| Characteristics | All subjects, n=96 | 0–18 years, n=8 |
|---|---|---|
| Female, n (%) | 68 (70.8) | 8 (100.0) |
| Age, mean (years) | 35.0±14.7 | 17.0±1.4 |
| Weight, mean (kg) | 82.1±29.4 | 56.5±7.7 |
| Co-ingestion agents per subject, n (%) | Mean 2.24 | Mean 0.5 |
| Opioids | 31 (32.3) | 0 (0.0) |
| Benzodiazepines | 18 (18.8) | 0 (0.0) |
| Ethanol | 11 (11.5) | 1 (12.5) |
| Diphenhydramine | 8 (8.3) | 0 (0.0) |
| Marijuana | 8 (8.3) | 0 (0.0) |
Table 2
| Type of ingestion, n=96 | Number (%) |
|---|---|
| Acute, known time | 56 (58.3) |
| Appropriate per RMN | 20 (35.7) |
| First serum APAP levels high after 4 hours of ingestion | 15 (75.0) |
| First serum APAP level high before 4 hours of ingestion but subsequent level above threshold for treatment | 5 (25.0) |
| Inappropriate per RMN | 36 (64.3) |
| Serum APAP levels before 4 hours | 21 (58.3) |
| Serum APAP levels below treatment threshold | 15 (41.7) |
| Chronic >24 hours | 17 (17.7) |
| Unknown time | 23 (24.0) |
| High serum APAP level, per RMN | 5 (21.7) |
| Low serum APAP level, per RMN | 18 (78.3) |
RMN, Rumack-Matthew Nomogram; APAP, acetaminophen.
IV NAC initiation was concordant with the Rumack-Matthew Nomogram for acute APAP toxicity in 20 of 56 subjects (35.7%) who presented with a known time of ingestion (Table 2). IV NAC was initiated to treat high serum APAP concentrations that were drawn prior to four hours post ingestion in n=21 (35.5%) of subjects with acute toxicity. However, n=5 (8.9%) subjects had a subsequent serum APAP concentration drawn appropriately that was above the hepatotoxicity line. These were not included in the financial analysis as inappropriate treatments. Fifteen (26.8%) initial serum APAP concentrations were below the possible hepatotoxicity line and did not warrant administration of IV NAC (Figure 2).
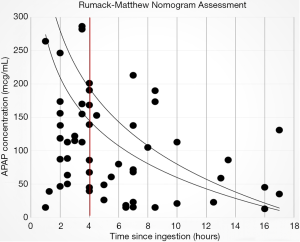
Twenty-three subjects had unknown time of exposure. Five (21.7%) of them had serum APAP concentrations above the possible hepatic toxicity line four or more hours after admission, while n=18 (78.3%) did not have serum concentrations above the hepatotoxicity line or did not have further APAP serum concentrations drawn after initiation of IV NAC.
Based on our hospital’s GPO pricing, the pharmacy experienced $11,352 in excess expenditure from administering IV NAC inappropriately. A breakdown of inappropriate use is listed in Table 3. The healthcare cost for these 96 hospitalizations totaled $1.8 million. There was a potential saving of $253,891.85 in healthcare costs (14% of total cost) if IV NAC initiation had followed accepted guidelines. This cost included facility charges, laboratory test charges, medication charges, and physicians’ charges, but did not include emergency room charges as those were unavoidable costs for all subjects.
Table 3
| Potential cost savings from inappropriate IV NAC | Cost (total $11,352.00) |
|---|---|
| NAC given with RMN below hepatoxicity line | $6,192.00 |
| NAC given based on serum concentration <4 hours | $4,214.00 |
| Dose exceeds manufacturer labeling | $946.00 |
RMN, Rumack-Matthew Nomogram; IV NAC, intravenous N-acetylcysteine.
Of the 96 subjects treated for APAP toxicity, n=12 (12.5%) received more than three doses of IV NAC. The most common reasons for extension of therapy were persistently elevated hepatic transaminase levels, detectable APAP serum concentration prior to discontinuation at 21 hours, or delayed time to presentation for treatment. Those were considered appropriate indications for continuing treatment. Of the 346 total doses of IV NAC administered throughout the study, n=47 (13.6%) exceeded manufacturer maximum suggested recommendations. The higher doses were all related to subjects with body weight higher than 100 kg.
Two (2.1%) subjects experienced an adverse reaction while receiving IV NAC. One of the reactions was anaphylaxis, which resolved after treatment with steroids and diphenhydramine, and the other was shortness of breath that resolved after discontinuation of IV NAC. Nine subjects (9.4%) experienced transaminitis with either aspartate or alanine aminotransferase greater than 1,000 international units per liter while receiving IV NAC. Only 2 (2.1%) were transferred to a liver transplantation center due to fulminant liver failure. One (1%) subject died as a result of liver failure.
Discussion
The financial impact of inappropriate initiation of IV NAC over four years resulted in a potential waste of $253,891.85. This could have been theoretically saved by following the current guideline and treatment regimen. Healthcare providers should be mindful of potential harm to patients when deviating from standard guidelines, including the increased cost incurred. These increased costs are important to both patients and healthcare systems, each of whom share a vested interest in keeping costs low while maintaining optimal patient outcomes.
The majority of subjects (n=56, 58.3%) in our study received IV NAC due to acute, known time APAP ingestion, however, unknown time of ingestion was a problem for n=23 (23.4%) of subjects. The potential savings opportunities can be found in decreasing the unnecessary hospital admissions for acute toxicity, which accounted for close to 14% of the treatment cost of all APAP toxicity cases. In this study, the average subject had a co-ingestion of two other medications. In those cases, the hospitalization cost nor the initiation of IV NAC were considered inappropriate, as it is difficult to predict the effects of multiple ingestions on liver function. All subjects with chronic APAP toxicity (n=17, 17.3%) had appropriate initiation of IV NAC based upon the detection of APAP serum concentration and elevated liver enzymes at least twice the normal levels.
The current, widely accepted treatment regimen for APAP toxicity was developed in the UK in the 1970s, with minimal changes in regimen since (6). The Rumack-Matthew Nomogram is applicable after the fourth hour of ingestion and is not valid for patients who present beyond 24 hours after an acute overdose (13), patients with an unknown time of ingestion, patients with a history of a staggered overdose, and patients with a history of repeated supratherapeutic ingestion. In this study, we were not able to determine the reasoning for poor concordance to the guidelines, but we expect it to be related to providers’ concerns of potential liver injury, especially when the providers feel obligated to address high APAP serum concentrations obtained prior to the full four hours after ingestion (Figure 2). It is not clear to us if this is due to poor trust of the nomogram, lack of trust for patients’ claimed timeline of ingestion, or lack of awareness of the need for at least 4 hours prior to obtaining APAP serum concentrations to initiate treatment. It is imperative that providers continue to adhere to well-established, standardized guidelines for treatment of APAP toxicity. Our hospital system elected to use this one bag system of 400 mg/kg due to the reported benefits of better tolerance, effectiveness, less interruptions, and fewer compounding errors compared to the 300 mg/kg multi-bag system (5).
Prevention of hepatic injury that can lead to fulminant liver failure secondary to acute APAP toxicity is more readily achievable with the approval of IV and enteral NAC formulations. While no randomized controlled trials exhibiting hepatoprotective effects of NAC exist, it has been shown in observational studies to improve outcomes in individuals when initiated within eight hours of toxic APAP ingestion (3). The advantages of utilizing IV NAC include a shorter duration of therapy and decreased incidence of nausea compared to enteral formulations. Duration of therapy is an important difference between the enteral and intravenous formulations of NAC (14). This analysis only included subjects who received IV NAC; therefore, it is not appropriate to extend the same conclusion to enteral NAC. Both IV and enteral NAC are effective for prevention of hepatotoxicity related to APAP overdose (10). Because this analysis did not include subjects receiving only oral NAC treatment, predicting if a similar problem exists with time of treatment initiation and adherence to guidelines is not possible.
Duration of treatment varies depending on the route of medication administration, APAP detection in blood samples at the end of the treatment, and the downward trend of hepatic transaminases. Although some authors reported low risk of prematurely discontinuing NAC treatment before the recommended 21 hours (9), in other instances, the treatment will need to be extended beyond the 21 hours. Evidence from one study favored a 72-hour NAC treatment course, regardless of the route of administration, compared to the historical 21-hour IV regimen for subjects with persistently elevated ALT (15). It is imperative to consider the cost on the healthcare system with extended hospitalization beyond what is proven to be an effective regimen, while maximizing patient outcomes. Providers should also consult with local Poison Control Centers, especially when questions arise regarding deviation of standard therapy.
Major limitations of this study include sample size, retrospective single institution study design, and lack of comparator group. It is also important to consider that some of our subjects were started on NAC treatment before the 4-hour level was obtained, possibly meeting treatment guidelines at the 4-hour mark, and not have been considered inappropriately treated. Despite this, we elected to count these individuals in the group of inappropriate use of NAC because treatment with NAC will cause unreliability of the subsequent acetaminophen levels. This could have inflated the potential savings if any of them did indeed need the treatment. Furthermore, although no data elements were missing from the records, due to the retrospective design, clinical findings were only able to be assessed based on documentation within our institution. Documentation could not be followed up on for subjects who were transferred to another facility. Despite these limitations, to our knowledge, this is the first study to address the potential healthcare cost savings that could be achieved by adhering to the currently accepted treatment strategies for APAP toxicity. A prospective, multicenter analysis comparing IV NAC to enteral formulations of NAC, with assessment of clinical outcomes would provide better insight into cost savings for a similar academic healthcare system. Furthermore, healthcare systems can use this information to enhance quality improvement at their institutions regarding education and adherence to current APAP toxicity guidelines.
Conclusions
The results from this study shows a high financial cost to the healthcare system when providers inappropriately initiate IV NAC for acute APAP ingestion. Following the administration guidelines of IV NAC may decrease overall expenses and potential adverse effects. There is a cost savings opportunity for individual hospitals and the entire healthcare systems by employing efforts to maximize appropriate use of IV NAC for subjects with acute APAP toxicity. A careful review of pertinent laboratory values and collaboration with regional Poison Control Centers to determine treatment duration may also be warranted.
Acknowledgments
This work had been conducted in the University of Missouri-Columbia system.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/jhmhp-20-87
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jhmhp-20-87
Peer Review File: Available at http://dx.doi.org/10.21037/jhmhp-20-87
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp-20-87). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The authors assert that all procedures contributing to this work comply with the ethical standards of the ICMJE national guidelines on human experimentation and has been approved by the appropriate committees at our institution (IRB approval #2006537). Given the nature of this study, the institution review board/ethics committee did not require HIPAA Authorization, Assent, and Parental Permission under Exempted criterion.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heard K, Green J. Acetylcysteine therapy for acetaminophen poisoning. Curr Pharm Biotechnol 2012;13:1917-23. [Crossref] [PubMed]
- Manthripragada AD, Zhou EH, Budnitz DS, et al. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf 2011;20:819-26. [Crossref] [PubMed]
- Prescott LF. Treatment of severe acetaminophen poisoning with intravenous acetylcysteine. Arch Intern Med 1981;141:386-9. [Crossref] [PubMed]
- Tsai CL, Chang WT, Weng TI, et al. A patient-tailored N-acetylcysteine protocol for acute acetaminophen intoxication. Clin Ther 2005;27:336-41. [Crossref] [PubMed]
- Johnson MT, McCammon CA, Mullins ME, et al. Evaluation of a simplified N-acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother 2011;45:713-20. [Crossref] [PubMed]
- Rumack BH, Peterson RC, Koch GG, et al. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med 1981;141:380-5. [Crossref] [PubMed]
- Daly FF, Dart RC, Prescott LF. Accidental paracetamol overdosing and fulminant hepatic failure in children. Med J Aust 2000;173:558-60. [Crossref] [PubMed]
- Chiew AL, Gluud C, Brok J, et al. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev 2018;2:CD003328 [PubMed]
- Lucyk SN, Yarema MC, Sivilotti ML, et al. Outcomes of Patients With Premature Discontinuation of the 21-h Intravenous N-Acetylcysteine Protocol After Acute Acetaminophen Overdose. J Emerg Med 2016;50:629-37. [Crossref] [PubMed]
- Green JL, Heard KJ, Reynolds KM, et al. Oral and Intravenous Acetylcysteine for Treatment of Acetaminophen Toxicity: A Systematic Review and Meta-analysis. West J Emerg Med 2013;14:218-26. [Crossref] [PubMed]
- Williamson K, Wahl MS, Mycyk MB. Direct comparison of 20-hour IV, 36-hour oral, and 72-hour oral acetylcysteine for treatment of acute acetaminophen poisoning. Am J Ther 2013;20:37-40. [Crossref] [PubMed]
- American College of Medical Toxicology. ACMT Position Statement: Duration of Intravenous Acetylcysteine Therapy Following Acetaminophen Overdose. J Med Toxicol 2017;13:126-7. [Crossref] [PubMed]
- Yarema MC, Green JP, Sivilotti ML, et al. Can a serum acetaminophen concentration obtained less than 4 hours post-ingestion determine which patients do not require treatment with acetylcysteine? Clin Toxicol (Phila) 2017;55:102-8. [Crossref] [PubMed]
- Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm 2006;63:1821-7. [Crossref] [PubMed]
- Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther 2012;342:529-40. [Crossref] [PubMed]
Cite this article as: Dalabih A, Cox C, Anderson J. Cost of inappropriate use of intravenous N-acetylcysteine for acetaminophen toxicity. J Hosp Manag Health Policy 2021;5:26.