
Wearable technology to track activity and distress in breast cancer patients: case series
Introduction
Cancer is the second leading cause of mortality in the United States, next to heart disease (1), and breast cancer is the most common type of cancer among women (2). According to the American Cancer Society’s 2019 annual report, over 270,000 new cases of breast cancer occurred in 2018, with more than 41,700 women dying from this disease (3).
Cancer treatments have improved significantly over the last 40 years, with improved outcomes as measured by 5-year overall survival rates. Today, the 5-year overall survival rate for breast cancer exceeds 90% for localized disease and up to 99% if detected at an early stage (4). With these high survival rates, there are estimated to be more than 3.8 million breast cancer survivors in the United States (5). As the numbers of patients and survivors increases, it can be challenging for healthcare systems and providers to ensure these patients receive adequate and timely support.
Cancer patients often have a long and difficult treatment journey, with many possible complications along the way. According to the National Comprehensive Cancer Network (NCCN), approximately one-third of cancer patients experience significant distress which can impact the strength and ability to fight the disease, cope with the disease, and to follow the recommended course of treatment (6). Minor issues can turn into major complications when patients become overwhelmed, leading to unnecessary trips to the emergency department. Our team’s participation in the Oncology Care Model (OCM) demonstration project suggests that the top five reasons for emergency department visits by cancer patients, include: sepsis (16.9%); pneumonia (9.7%); urinary tract infection (UTI) (8.8%); nausea with vomiting (8.8%); and chest pain (7.8%) (7). Furthermore, the literature suggests that putting mechanisms in place to close gaps in care continuity post-discharge (i.e., in the primary or specialty care environment) could reduce overall healthcare utilization, including reducing emergency department visits (8).
Cancer patients and survivors may also exhibit a broad range of mild to moderate cognitive deficits in attention, information processing, verbal and visual capacity, long-term and working memory, spatial skills, command of language, and general motor functioning (9). Despite studies suggesting that patient navigation support lowers costs and improves the patient experience (10,11), the complex nature of cancer treatment often results in poorly-managed hand-offs between care team providers and inadequate overall psychosocial support, and can lead to survival rates being impacted, especially where these services are not integrated (12).
Often, care team members and patients do not realize the degree to which patient needs go unrecognized. This leads to issues along the healthcare delivery continuum that may hinder the ability to recover and lead a healthy life. When this occurs, an issue or complication that could have been more easily resolved, if addressed early, can become a significant concern for which the patient seeks care in the emergency department.
One mechanism for identifying, and thus addressing, such issues earlier in the healthcare delivery continuum is to include patient self-reporting vehicles, such as wearable technology, into healthcare delivery models. Doing so allows for data capture at the most proximal point in distance and time to augment communication, increase care coordination, increase appropriate information delivery, and decrease emergency department utilization.
As such, the primary aim of this project was to illustrate the potential value of integrating into a breast cancer navigator program, near real time, patient and wearable device reported data from a patient perspective and a navigator perspective. This case is unique because it includes use of a distress thermometer with the use of wearable device data as opposed to assessing the value of the wearable by itself.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jhmhp-20-99) (13).
Methods
This project, named “Wear2Care,” used a mixed methods case approach to understand the degree to which proximal monitoring of patient and wearable reported data contributed the overall patient experience and to care coordination. The case approach was appropriate because such an approach allows for an uncontrolled study design (14), descriptive and narrative reporting (15) and accepts a small participant unit, such as a few participants, as a demonstration on the way to a larger study (14,15).
In terms of quantitative methods, study participants were provided with a Fitbit Alta HR to track steps, heart rate, and sleep. In terms of qualitative methods, study participants performed daily tracking of distress levels using a paper Distress Thermometer (6) (Figure 1) instrument and semi-structured focus groups were conducted with patients and navigators. An integrated, third party database management system, Fitabase, was used to collect the data points used in this study. Fitabase provided real-time dashboards of each patient’s activity, as well as cumulative data points in a secure, online database. Aggregate data were downloaded and analyzed using Microsoft Excel. This study was conducted between January and March 2018 for a rolling 28-day period. This study underwent review by the University of Alabama at Birmingham (UAB) Cancer Protocol Committee where it was determined to be a quality improvement project and therefore not suitable for Institutional Review Board (IRB) review. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Each participant was given full disclosure of the purpose and process of the Wear 2 Care program. Each participant gave their informed consent to participate in the program, as well as for the researchers to have electronic access to each participant’s individual Fitbit data. Participants set up their own online Fitbit account and gave their password to their navigator, who was able to access and assist participants with the study. At the conclusion of the 28-day study term, each participant was reminded to change their account password to ensure privacy.

Twenty patient participants were recruited from among the academic medical center’s cancer center. Participants included the population of patients diagnosed with breast cancer (stages 0, I, II, III) who were actively receiving, or who had recently received, breast cancer treatment and care. After ascertaining that the participant had not previously used any type of health tracking device, besides a pedometer, each participant was provided with a Fitbit Alta HR fitness tracker and instructed on how to wear and synchronize it regularly. The participants were asked to complete several tasks throughout the pilot project. These tasks included:
- Wearing their Fitbit during both waking and sleeping hours;
- Downloading and setting up the Fitbit mobile app, and managing it for the 28-day study period;
- Charging and synchronizing the Fitbit as needed;
- Measuring their distress levels every day using a paper Distress Thermometer instrument, and
- Participating in a focus group after wearing the Fitbit for 28 days.
Navigator participants were recruited from a group of certified exercise physiologists who were trained and assigned as navigators to work with the participants. This was deemed to be an appropriate group because of their comprehensive expertise in health, exercise, and general wellbeing. Navigators used standard Fitbit recommended baseline data as an indicator for measuring deviations in terms of steps, physical activity, and sleep.
The navigators were trained on protocols for escalating significant adverse events, as defined by the patient as potentially leading to an emergency room visit, unplanned hospitalization, or other unexpected adverse health complication. In the absence of such an adverse event, navigators were instructed to engage with patients in discussions about their health status, encourage healthy lifestyle choices, and document patient experience comments about the use of the Fitbit tracker.
In all, 20 patients were recruited and enrolled. Nineteen (95%) finished the study, resulting in 19 participants times 28 days each, for in a total possible 532 combined Fitbit data collection days. Only 14 of the participants (70%) collected daily distress data, resulting in a total possible 392 data collection days for distress analysis. The navigation team participated in a focus group separate from patient participants. All data were analyzed using Microsoft Excel®. Table 1 shows the distribution of all 20 participants, although only 19 of those were used in the final analysis.
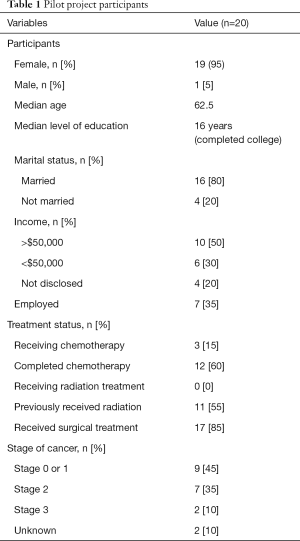
Full table
Findings
In Figure 2, we show the pathway of the enrollment and data collection through both quantitative and qualitative methods followed by a summary of the findings. Details behind the findings are then explained first by quantitative followed by qualitative. Focus group findings are further divided by participants and navigators.
Quantitative findings
In terms of participant data, out of the total possible 532 data collection days, Fitbit activity data were accurately collected for 487 days (91.5%), and distress assessment data were accurately collected for 338 days (73.7%). The strongest positive correlations were found between minutes asleep and number of awakenings (r=0.6852), steps and minutes lightly active (r=0.6736), and steps and minutes very active (r=0.6149). The strongest negative correlations were found between distress levels and minutes fairly active (r=−0.3346), steps and minutes sedentary (r=−0.4089), and minutes sedentary and minutes lightly active (r=−0.4086). See Table 2 for all correlations. Although the sample size was small, a positive correlation between minutes asleep and number of awakenings (r=0.6852), and a negative correlation between distress levels and minutes fairly active (r=−0.3346), stood out as potential indications of the impact of distress on breast cancer patients.
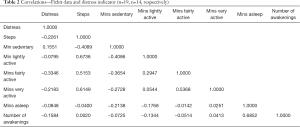
Full table
Focus group findings: patient information
Key focus group findings include participants reporting that knowing their step counts and their sleep data were two of the primary benefits of wearing the Fitbit. Several participants stated that they utilized the calorie tracking feature of the accompanying Fitbit app. One participant represented the comments of others by saying: “The Fitbit motivated me to move, exercise, and be more active.” Some participants followed in agreement that they had been able to lose weight during the program, with the highest amount of weight loss being 12.3 pounds over a 28-day period of use. Several participants also shared that another, albeit unexpected, benefit of the Fitbit was that it provided them with hope. One person, summing up the expressions of several, said, “The Fitbit gave me something to work towards, to keep moving forward and living life.”
Several participants shared that wearing the Fitbit and participating in the Wear2Care program was a positive influence on their quality of life, particularly regarding being more active and having gentle reminders to exercise. Others stated that there had been no impact on their quality of life. Every participant voiced approval for the concept of sharing their information with healthcare providers and felt that having it incorporated into treatment plans was important and made a difference.
Some potential concerns brought up by the participants included feelings of frustration and discouragement from not being able to meet the fitness goals suggested to them from their Fitbit. Even so, the participants expressed excitement about the potential care quality benefits and improved conversations they could have with their care providers, if they were able to see their Fitbit data and discuss it together.
Focus group findings: navigator information
The navigators indicated that remote monitoring of the patient’s status was beneficial, and allowed them to have a more comprehensive and holistic view of their patients. Currently, navigators only interact with patients when they come to the hospital, or during a phone call, and the navigators expressed interest and value in being able to see the patients’ data to visualize how their patients are doing over time. Navigators also shared that the distress level data and the sleep data were particularly beneficial, as they mentioned that many of their patients have trouble sleeping. They thought that seeing the patterns of sleep disruption, coupled with slowing activity, may provide better alerts as to when escalated intervention may be necessary.
An additional benefit to having patient reported data was the improved quality of conversations navigators can have with their patients. They emphasized that the continuously reported data enhanced their ability to have meaningful, effective, and time appropriate discussions. As such, navigators felt that these data provide a level of richness that facilitated accelerated intervention of established pathways. They explained that this would better equip them to encourage patients to talk about their goals of care relative to how cancer is affecting their life. They also stated that connecting the Fitbit data to the patient’s electronic health record, so that the data could be shared, would be beneficial to the entire care team.
Discussion
The purpose of this project was to understand how the use of self-reported data could enhance patient-centered breast cancer care through the collection of wearable tracker data and completion of the daily distress indicator. It also sought to understand the value of these data to the patient care navigators for care transitions. Overall, participants found the Fitbit to be beneficial, regardless of their status of cancer therapy or recovery. More specifically, the collective of findings (i.e., patients and navigators) align well with the current literature pointing toward improved overall patient experience with effective and appropriate navigation support (10,11). For example, participants felt that sharing their information and incorporating it into the treatment plans made a difference. Participants also shared the perception of feeling hope. Additionally, navigators felt that the remote monitoring provided a more comprehensive and holistic view of their patients leading to improved conversations with the patient.
Collectively, the findings suggest that the combination of the wearable tracker and the distress indicator provided value to both participant groups. As this was a case study, this represents an area of more rigorous research and development in wearable trackers as a search of the literature indicates no studies have yet been published reporting on the combination.
In light of the overarching finding that wearables that include the distress indicator could provide a level of benefit not yet studied, this study has several limitations. First, this was conducted as a feasibility and process improvement project. Although case reports allow for a small number of participants, this, together with a small sample of participants (n=20) not necessarily being representative of the larger population of breast cancer patients, contributes to limited generalizability. Because of the original intent as a feasibility and process improvement project, pre-intervention data were not collected. Additionally, relying on focus group reports introduces the potential for natural biases inherent in group conversation, such as stressed statements, fear of speaking up in front of the study investigators, personality types, poor communication skills, and other biases. Lastly, the CARE Guidelines (13) were not initially considered in the study design. While this might limit the rigor of the study design, it does not necessarily translate to limited findings, given our focus.
Conclusions
This project affirmed the beneficial potential of incorporating wearable data into treatment plans for patient-centered breast cancer care, as well as insight on the strength of the relationship between distress levels and patient fitness activity and sleep quality. The use of wearable technology was found to enhance the timeliness of receiving patient information, enabled accelerated intervention of established care pathways, and enhanced patient engagement and experience. Technology-enabled patient navigators will be better equipped to help patients receive the appropriate support at the appropriate time and in a manner that is easily understood. Using patient reported outcomes like these, patient navigators are empowered to determine when and how to interact with their patients to ensure proper patient-centered care coordination, determine wellbeing, improve healthcare delivery, and potentially minimize symptom escalation.
Acknowledgments
Funding: Funding for this project was provided by the Deep South Cancer Foundation, Birmingham Alabama, a 501(c)3 public charity, providing funding for cancer research and patient care activities.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Naleef Fareed, Ann Scheck McAlearney, and Susan D Moffatt-Bruce) for the series “Innovations and Practices that Influence Patient-Centered Health Care Delivery” published in Journal of Hospital Management and Health Policy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jhmhp-20-99
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp-20-99). The series “Innovations and Practices that Influence Patient-Centered Health Care Delivery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilligan AM, Alberts DS, Roe DJ, et al. Death or Debt? National Estimates of Financial Toxicity in Persons with Newly-Diagnosed Cancer. Am J Med 2018;131:1187-1199.e5. [Crossref] [PubMed]
- Centers for Disease Control and Prevention [Internet]. U.S. Cancer Statistics Stat Bites 2020. Available online: https://www.cdc.gov/cancer/uscs/about/stat-bites/index.htm
- American Cancer Society [Internet]. Cancer Facts & Figures 2019. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- American Cancer Society [Internet]. Cancer Facts & Figures 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- Breast Cancer Research Foundation [Internet]. Breast Cancer Statistics and Resources 2020. Available online: https://www.bcrf.org/breast-cancer-statistics-and-resources
- National Comprehensive Cancer Network [Internet] NCCN Distress Thermometer 2020. Available online: https://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf
- Hsieh VC, Hsieh ML, Chiang JH, et al. Emergency Department Visits and Disease Burden Attributable to Ambulatory Care Sensitive Conditions in Elderly Adults. Sci Rep 2019;9:3811. [Crossref] [PubMed]
- Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ 2017;356:j84. [Crossref] [PubMed]
- Lindner OC, Phillips B, McCabe MG, et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 2014;28:726-40. [Crossref] [PubMed]
- Rocque GB, Partridge EE, Pisu M, et al. The Patient Care Connect Program: Transforming Health Care Through Lay Navigation. J Oncol Pract 2016;12:e633-42. [Crossref] [PubMed]
- Williams M, Nielson D, Dayao Z, et al. Patient reported measures of a breast cancer nurse navigator program in a minority, rural, and economically disadvantaged patient population. Cancer Res 2020;80:abstr P6-11-10.
- Clauser SB, Wagner EH, Aiello Bowles EJ, et al. Improving modern cancer care through information technology. Am J Prev Med 2011;40:S198-S207. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep 2013;7:223. [Crossref] [PubMed]
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60-3. [Crossref] [PubMed]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet 2002;359:145-9. [Crossref] [PubMed]
Cite this article as: Smedley W, Feldman SS, Ralphs C. Wearable technology to track activity and distress in breast cancer patients: case series. J Hosp Manag Health Policy 2021;5:21.


