
Barriers and facilitators to hospital implementation of obstetric emergency safety bundles: a qualitative study
Introduction
Maternal mortality is rising in the United States, with recent estimates suggesting an incidence of 22 out of 100,000 live births (1); trends suggest that there is an even greater incidence of maternal morbidity and complications (2-10). In recognition of the seriousness of this problem, reducing maternal mortality was incorporated as a specific objective into both the United Nation’s Millennium Development Goals and the Healthy People 2020 (11). Many of the causes contributing to maternal morbidity and mortality are preventable, yet approaches to managing the safety of care for women giving birth remain inadequate.
Notably, three obstetric conditions account for nearly a third of all pregnancy related mortality and morbidity, and each can be prevented with appropriate resources and planning. These three conditions are post-partum hemorrhage (PPH), preeclampsia (PE), and venous thromboembolism (VTE). The development and implementation of safety bundles is a key approach that can potentially improve outcomes for these conditions. Safety bundles are small sets of evidence-based independent interventions, such as checklists, protocols, or education materials, that work together synergistically to improve outcomes (12). Safety bundles have proven effective at reducing morbidity and mortality in several healthcare conditions (13,14), and there is emerging evidence that these bundles are an effective way to reduce the adverse events associated with PPH, PE, and VTE (15-17). Critically, implementation of all of the elements of the bundle is essential to its success, and there remain several barriers that can jeopardize the effectiveness of safety bundles (12,15).
This study aims to assess and document the facilitators and barriers that are associated with implementing obstetric emergency safety bundles targeting PPH, PE, and VTE. Our goal is to provide evidence about common pitfalls in the implementation of these safety bundles in U.S. hospitals. Better understanding of the facilitators and barriers to safety bundle adoption and use can support future implementation and dissemination efforts focused on reducing maternal mortality and improving the quality of maternity care. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jhmhp-20-74).
Methods
Context
Merck for Mothers, a philanthropic program initiated by Merck, established a 10-year, $500 million program to address maternal mortality worldwide (18). As part of this program, Merck for Mothers funded three separate Quality Improvement Collaboratives (QICs) to develop and disseminate obstetric emergency safety bundles and support their implementation: The American Congress of Obstetricians and Gynecologists (ACOG) District II Safe Motherhood Initiative (SMI) (16); the Association of Women’s Health, Obstetric, and Neonatal Nurses (AWHONN) Postpartum Hemorrhage Project (PHP) (17,19); and the California Maternal Quality Care Collaborative (CMQCC) California Partnership for Maternal Safety (CPMS) (6,20-22). Merck for Mothers provided the QICs with funding, but was not involved in the design or conduct of their projects.
Each QIC supported the implementation of the safety bundles either in isolation or in combination with hospitals within their provenance (see Table 1). Across QICs and for all three conditions, the safety bundles utilized a Readiness, Recognition and Prevention, Response, and Reporting framework to standardize care in the event of PPH, PE, or VTE (23-25). Each bundle incorporated human factors (e.g. , posting early warning signs, team huddles, and debriefs), education (e.g. , computer-based learning modules), and clinical practice changes (e.g. , use of algorithms, protocols, and checklists pertinent to the specific condition targeted). The three QIC projects ran concurrently from May 2013 to December 2016, and solicited voluntary participation from hospitals. However, each QIC project was administrated separately and had different safety bundle implementation strategies.

Full table
Study design
The focus of our study was to identify facilitators of and barriers to safety bundle implementation that managers in the hospitals participating in the QICs could control. Using a multiple case study design, we held semi-structured interviews with representatives of each QIC and from participating hospitals. This approach enabled an in-depth assessment of the process of implementing the safety bundles in a range of hospital contexts. Our study aimed to synthesize lessons learned across participants at hospitals within each QIC rather than compare differences in the strategies deployed by the QICs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was determined to be exempt from Institutional Review Board approval due to its minimal risk and focus on quality improvement. Informed consent was taken from all study participants.
Data collection
QIC interviews
Using a purposeful sampling approach, we conducted semi-structured one-on-one and group interviews with all of the identified personnel at each of the three QICs. A lead investigator and an additional investigator facilitated in-person interviews or telephone interviews, when necessary. The interview guide for the QICs asked questions about the project goals and approaches to implementation (e.g. , What is the goal of your organization in implementing the obstetric emergency safety bundles? What has been your relationship with the hospitals throughout the implementation process?) (Interview Guide available upon request). In total, we conducted 28 interviews across all three QICs. All interviews were recorded and transcribed verbatim.
Hospital interviews
The project administrator at each QIC was asked to nominate four higher- and two lower-performing hospitals with respect to their implementation of the safety bundles for further study of the implementation process. QICs made their nominations based on implementation data that they had collected and perceptions about the hospitals’ levels of engagement with the program. We also attempted to achieve variability across hospitals studied with respect to system membership, size, and urban location. As a result, CMQCC nominated a fifth higher-performing hospital that was representative of a system member hospital in a rural area.
We interviewed the project lead(s) at each of the nineteen nominated hospitals (n=25). Project leads included nurses, clinical nurse specialists (CNSs), and physicians. A summary of hospital interviewees is provided in Table 2. Interviews occurred via telephone, and were facilitated by a lead investigator and supported by an additional investigator. The interview guide asked questions regarding the facilitators (e.g. , What factors have been helpful in the implementation of the safety bundles?) and barriers to implementation of the bundle(s) (e.g. , What barriers or challenges have you faced in the implementation of the safety bundles?), resources and support that were needed for implementation (e.g. , What resources have been made available in the implementation process?), practice changes associated with the bundles (e.g. , How have you incorporated the safety bundles into your workflow? Has anything needed to change?), the data collection process (e.g. , Can you tell us about how data about the project was collected?), and the ongoing status and sustainability of bundle use (e.g. , Do you feel that the safety bundles are used whenever necessary?) (Interview Guide available upon request). All hospital interviews were recorded and transcribed verbatim.

Full table
Data analysis
Across interviews we iteratively read interview transcripts and discussed findings as the study progressed. This constant comparative approach enabled us to explore emergent themes and to ensure that we reached thematic saturation in data collection from the QICs and the hospitals (26). We embraced grounded theory principles (27) in that we collected and analyzed data simultaneously to maximize our ability to explore emergent themes.
We initially employed an inductive approach to analyze the QIC and hospital interviews. A coding team directed by the lead investigator—an experienced qualitative researcher—created a preliminary coding dictionary defining broad categories of findings from the transcripts. The coding team also included a second experienced qualitative researcher, and a junior researcher. Data were further classified from the broad codes into themes (28). Coders met periodically throughout the coding process to resolve discrepancies and ensure consistency through discussion and negotiation (29). We also reviewed any new codes or themes that emerged, consistent with a grounded theory approach (27,30). We used the ATLAS.ti software program (31) to support the coding process.
Results
Our analysis revealed several facilitators and barriers to hospitals’ implementation of the obstetric safety bundles. Notably, the facilitators and barriers were similar in hospitals across the three QICs, and across the bundles for PPH, PE, or VTE. Additionally, we found that the difference between the higher- and lower-performers was rarely due to the presence (or absence) of a particular characteristic, but rather higher-performers often experienced the same barriers yet demonstrated a greater ability to overcome the challenge.
Barriers
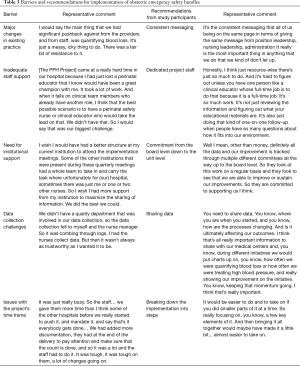
We found four common barriers to obstetric safety bundle implementation: (I) major changes in existing practice were required; (II) inadequate staff and institutional support; (III) data collection challenges; and (IV) issues with the project’s time frame (see Table 3).
Full table
Major changes in existing practice were required
The bundles each required changes to clinical practice that were noted as challenges. This barrier was most prevalent for the PPH bundles that were required to use the metric of quantified blood loss (QBL) rather than estimated blood loss (EBL). This requirement was established as a component of the PPH bundle implemented by hospitals in all three QICs as it enabled hospitals to accurately measure blood loss and thereby appropriately assess risk for PPH. However, as a nurse at one hospital explained, this change required additional work during delivery, “They’re thinking, ‘How are we going to do [QBL] when we are doing all the…’ you know, it’s that whole juggling of duties and it’s more work. ”
In addition to the increased work that QBL introduced, it also raised the issue of introducing physicians to a QBL measurement that may differ from their EBL value. For instance, a nursing quality and safety officer who oversaw 15 hospitals for a health system described this dynamic:
“I still think that we have a challenge of some OB (obstetric) physicians/providers and multiple anesthesia providers don’t truly believe in the numbers we give them. Because what we learned was when we quantify blood loss with Cesarean section, we actually are seeing much lower blood loss. So for example, surgeons will say in a routine C-section, a patient loses 800. We were measuring 300–500 mL (milliliters) in a routine C-section. So when we quantified and got those numbers, they didn’t believe us. ”
To overcome these challenges, several sites reported that reinforcement and consistent messaging helped to make QBL part of routine practice.
Inadequate staff and institutional support
Lack of staff support and staff turnover presented another barrier to safety bundle implementation. First, if there was no staff member to champion the quality improvement initiative, implementation was a challenge. An obstetric physician at one hospital described this dynamic: “And even though they [the anesthesia department] work with us in surgical emergencies on labor and delivery, we really, we didn’t have a leader from their group that was willing to collaborate with us. ” This gap could thus compound issues related to multidisciplinary engagement as clinicians from different specialties collaboratively work together to implement the bundles. In addition, many staff members mentioned that staffing issues such as having inadequate time to manage the quality improvement project and staff turnover (e.g. , losing project champions) created challenges that ultimately impacted their implementation timelines (see Table 3).
Related to the issue of staffing was the notion of the need for institutional support of the program. Some hospital administrators protected time for their quality improvement project staff, enabling them to better engage with the QIC and access the resources promulgated by the QIC. The QICs purposely required department administrators and directors to sign a letter offering institutional support prior to beginning the project. However, this support did not always translate to allocation of resources for the project. Some hospitals struggled to find sufficient time and resources to engage with the QIC, including having time for nurses to attend education sessions.
Data collection challenges
A critical part of the efforts by each of the three QICs was to collect and report data in order to evaluate the impact of their projects. This effort necessitated data reporting by all hospitals, and this activity proved to be a major challenge. Some hospitals made efforts to build tools in their electronic health record (EHR) in order to track patients with PPH or PE, but some EHRs were limited in how amendable they were. As a result, nursing staff often did manual chart reviews, which in addition to being time consuming, introduced concerns about the validity of the data. This latter issue about data validity manifested itself in other ways such as when the success of the project as a whole was questioned, as described by one physician: “I’m just a little concerned that we may not be seeing the whole story. Either we’re phenomenally successful, or we just don’t have all the data yet. I tend to be a bit cynical so I’m thinking that it’s probably the latter. ”
One approach mentioned by the interviewees that helped to motivate project participants to overcome their challenges with data entry was to share the data on an ongoing basis. Hospitals viewed sharing data as essential to maintain buy-in for the safety bundle implementation.
Issues with the project’s timeframe
Across QICs, the implementation of the obstetric safety bundles was also challenged due to constraints with the project’s timeline. This challenge was described by one hospital’s nursing lead:
“First of all, we had some fairly small numbers that we were working with. It’s like serious complications they were looking for. And then this was a totally brand new project. So it did take a while to figure out what we really wanted to look at. And then I think the fact that the project was so short-lived. I don’t think it gave us enough time to really see the trends and see the effects of all the things that were put in place. ”
Some hospital interviewees discussed that breaking down the implementation process into step-by-step pieces could make it feel more manageable and reduce staff concerns and perceptions about being overwhelmed by the bundle implementation project.
Facilitators
Our analysis revealed two common facilitators that were in the control of the hospital itself that helped with safety bundle implementation: training and education; and multidisciplinary engagement (Table 4).

Full table
Training and education
Training and education were viewed by hospitals as essential to transform care delivery. For instance, one nurse noted: “We felt that [the education modules] were well done and it really spoke to the ‘why. ’ And it kind of made everyone really aware from the start of what we were doing and why we were doing it. So it was really important to set that message from the get-go…” Importantly, training and education occurred via multiple formats: use of simulations and drills, online modules, inter-organizational learning, and grand rounds lectures. These different modalities expanded the opportunities for learning beyond a top-down approach from the QIC organizers to the participants and offered hospitals the ability to develop their own approaches to education.
In this vein, a motivated and engaged CNS was often viewed as essential for the success of the project because that staff member could lead and drive the education and training.
Multidisciplinary engagement
Hospitals viewed multidisciplinary engagement as collaboration between managers, physicians, nurses, pharmacists, and technicians from multiple departments and viewed this engagement as a critical element of what made safety bundle implementations effective. Furthermore, hospitals noted how tools supplied by the QICs helped to support multidisciplinary engagement efforts within their institutions. In particular, hospitals discussed how helpful debriefing tools were. One nurse explained, “The debriefing tool I think has been probably one of the best pieces, or best tool for me and for my staff, because we walk away, let’s say like our OB hemorrhage. You know with every OB hemorrhage, something different happens. And we use that tool to come together and talk about, you know, what went right, but also what went wrong, and then how could we improve. ”
A common challenge with multidisciplinary teams that focused on safety bundle implementation involved the lack of engagement by anesthesiologists: “I find that anesthesia providers are probably the more challenging group to really believe in this. I saw that in my own medical center and I continue to see that in our 15 medical centers. ” One approach that appeared to help garner buy-in from anesthesiologists was presenting evidence to them. As told by one hospital’s nurse lead: “I think when Dr. [XXX]… I can’t remember if there was an article, or something, that he brought to the anesthesiologist where he had identified that this is what should be done on every case, every case should be QBL not estimated…And then the acceptance by the physicians… And having them understand the benefit of doing it with all of our cases. ”
Discussion
Translating best practices in reducing maternal mortality and implementing new approaches to improving care across diverse contexts remains challenging. By examining facilitators and barriers to implementation of obstetric emergency safety bundles in hospitals that participated in QICs across five states, we aimed to identify lessons learned to support future hospital implementation efforts. Briefly, we found that major changes to existing practice, maintaining appropriate staffing, obtaining institutional support, reporting data, and managing project timelines are challenges to implementation that can be addressed by the hospital’s management. Importantly, overcoming these barriers is rarely accomplished with a single solution, but rather requires viewing the implementation holistically and employing a coordinated effort to incorporate the safety bundles into routine care. At the same time, training and education and multidisciplinary engagement were found to facilitate successful implementation of the safety bundles.
Significant attention and focus have been recently drawn to improving the care for women with PE, PPH, and VTE. For instance, several professional associations formed the National Partnership for Maternal Safety (NPMS)—a workgroup of the Council on Patient Safety in Women’s Health Care (24,32). NPMS developed safety bundles that included evidence-based standardized protocols and guidelines to address the obstetric emergency conditions (24). However, a recent survey found that only 67% of obstetric units utilized an available PPH safety bundle, despite the evidence (33). Moreover, that study also found the use of safety bundles was biased toward large, academic hospitals.
The findings we report can aide in hospitals’ efforts to standardize care around obstetric emergencies via safety bundle implementation. Previous work has identified similar barriers in the implementation of obstetric emergency safety bundles (20,34,35). Specifically, Vamos et al. examined the implementation of an obstetric PPH bundle in hospitals through a quality collaborative in Florida (34). That study identified critical determinants of practice including leadership and staff buy-in. However, our results demonstrate that these challenges were not idiosyncratic to those individual bundles and the states that they were examined within, but persist across different regions of the country and in multiple bundle implementations mediated by three distinct QICs. Our results additionally highlight that hospitals have management approaches at their disposal that can overcome identified barriers, which may be critical as QI efforts in maternal care expand nationally.
Our findings additionally offer insight into areas that QICs can specifically target to help support implementation (36). For example, our finding regarding the challenges related to institutional support suggests that the QIC may be well positioned to guide hospital leadership in evaluating the fit between the changes required to implement the bundle and their specific institutional practices and resources. Nonetheless, investigations into QICs to date have largely focused on the participants, with considerably less attention paid toward the multi-level interactions between the participants and the QIC (35). Our findings agree with previous research (37) in that understanding how QICs incorporate participant data and feedback to improve their operations will be essential to improve the efficacy of QICs.
Study limitations
Our study should be interpreted in light of several important limitations. This study sampled a small number of hospitals for each QIC, and a relatively small number of individuals within those institutions. Given the time and energy constraints of qualitative studies, there are significant barriers to large-scale studies. Similarly, our study cannot attest to the impact of safety bundle implementation on the processes and outcomes of care. Each QIC collected process metrics including the percent of safety bundle components adopted for each hospital, but this data was unavailable to our research team due to data use agreements between the hospitals and the QICs. We relied on the QIC knowledge of this data and their tacit knowledge of hospital engagement to help select hospitals for our further inquiry, but recognize that this selection could have been biased. Likewise, our study may be subject to non-response bias because some hospitals nominated by the sites did not respond to our solicitation to participate in the study. There are several sources of variation that could account for the barriers and facilitators we observed. Chief among these may be the design of the safety bundles offered by each QIC, which varied within condition and across sites. Exploring the sources of variation outside the managerial control of the hospital was beyond the scope of this current study, but remains an important area for further research. Additionally, given the timeframe of the quality improvement project, we were unable to evaluate sustainability of the use of the safety bundles. Reassessing the extent to which obstetric emergency safety bundles are used over extended periods of time is critical for all quality improvement efforts, and is another area for future research related to the obstetric emergency safety bundles.
Conclusions
While clinical approaches to preventing maternal mortality from PPH, PE, and VTE have been developed and packaged into safety bundles, less attention has been given to the implementation issues that hospitals may face. The detailed description and potential solutions to these challenges that this study reports can help hospitals realize the potential benefits of the safety bundle implementation. This study highlights key barriers and facilitators that hospital management can influence in order to improve the implementation of best practice approaches to standardizing care and preventing maternal morbidity and mortality.
Acknowledgments
Funding: The research in this manuscript was supported by funding from Merck, through its Merck for Mothers program, and is the sole responsibility of the authors. Merck for Mothers is known as MSD for Mothers outside the United States and Canada.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Hospital Management and Health Policy for the series “Innovations and Practices that Influence Patient-Centered Health Care Delivery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jhmhp-20-74
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jhmhp-20-74
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp-20-74). The series “Innovations and Practices that Influence Patient-Centered Health Care Delivery” was commissioned by the editorial office without any funding or sponsorship. ASM served as the unpaid Guest Editor of the series. DMW serves as an unpaid editorial board member of Journal of Hospital Management and Health Policy. Dr. DMW reports grants from Merck for Mothers, during the conduct of the study. Dr. ASM reports grants from Merck for Mothers, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was determined to be exempt from Institutional Review Board approval due to its minimal risk and focus on quality improvement. Informed consent was taken from all study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moaddab A, Dildy GA, Brown HL, et al. Health Care Disparity and Pregnancy-Related Mortality in the United States, 2005-2014. Obstet Gynecol 2018;131:707-12. [Crossref] [PubMed]
- Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol 2005;106:1228-34. [Crossref] [PubMed]
- Callaghan WM, Grobman WA, Kilpatrick SJ, et al. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol 2014;123:978-81. [Crossref] [PubMed]
- Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol 2010;202:353.e1-6. [Crossref] [PubMed]
- Grobman WA, Bailit JL, Rice MM, et al. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol 2014;123:804-10. [Crossref] [PubMed]
- Main EK, McCain CL, Morton CH, et al. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol 2015;125:938-47. [Crossref] [PubMed]
- Bingham D, Jones R. Maternal death from obstetric hemorrhage. J Obstet Gynecol Neonatal Nurs 2012;41:531-9. [Crossref] [PubMed]
- Shih T, Peneva D, Xu X, et al. The Rising Burden of Preeclampsia in the United States Impacts Both Maternal and Child Health. Am J Perinatol 2016;33:329-38. [PubMed]
- Tepper NK, Boulet SL, Whiteman MK, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol 2014;123:987-96. [Crossref] [PubMed]
- Troiano NH, Witcher PM. Maternal Mortality and Morbidity in the United States: Classification, Causes, Preventability, and Critical Care Obstetric Implications. J Perinat Neonatal Nurs 2018;32:222-31. [Crossref] [PubMed]
- US Department of Health & Human Services, Office of Disease Prevention & Health Promotion. Healthy People 2020. 2000.
- Arora KS, Shields LE, Grobman WA, et al. Triggers, bundles, protocols, and checklists--what every maternal care provider needs to know. Am J Obstet Gynecol 2016;214:444-51. [Crossref] [PubMed]
- Lindsay ME, Hovan MJ, Deming JR, et al. Improving hypertension control in diabetes: a multisite quality improvement project that applies a 3-step care bundle to a chronic disease care model for diabetes with hypertension. Am J Med Qual 2013;28:365-73. [Crossref] [PubMed]
- Baldelli P, Paciella M. Creation and implementation of a pressure ulcer prevention bundle improves patient outcomes. Am J Med Qual 2008;23:136-42. [Crossref] [PubMed]
- Main EK, Markow C, Gould J. Addressing Maternal Mortality And Morbidity In California Through Public-Private Partnerships. Health Aff (Millwood) 2018;37:1484-93. [Crossref] [PubMed]
- D'Alton ME, Chazotte C, Kulbida N, et al. Safe Motherhood Initiative Final Report: 2013-2016. Albany, NY: The American College of Obstetricians and Gynecologists, District II. 2017.
- Bingham D, Scheich B, Bateman BT. Structure, process, and outcome data of AWHONN’s Postpartum Hemorrhage Quality Improvement Project. J Obstet Gynecol Neonatal Nurs 2018;47:707-18. [Crossref] [PubMed]
- Merck for Mothers. Merck for Mothers working to end the tragedy of women in America dying in childbirth and pregnancy. 2013.
- Scheich B. Implementation and Outcomes of the AWHONN Postpartum Hemorrhage Project. J Obstet Gynecol Neonatal Nurs 2018;47:684-7. [Crossref] [PubMed]
- Main EK, Cape V, Abreo A, et al. Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. Am J Obstet Gynecol 2017;216:298.e1-e11. [Crossref] [PubMed]
- Main EK. Reducing Maternal Mortality and Severe Maternal Morbidity Through State-based Quality Improvement Initiatives. Clin Obstet Gynecol 2018;61:319-31. [Crossref] [PubMed]
- Main EK, Dhurjati R, Cape V, et al. Improving Maternal Safety at Scale with the Mentor Model of Collaborative Improvement. Jt Comm J Qual Patient Saf 2018;44:250-9. [Crossref] [PubMed]
- D'Alton ME, Friedman AM, Smiley RM, et al. National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Anesth Analg 2016;123:942-9. [Crossref] [PubMed]
- Main EK, Goffman D, Scavone BM, et al. National Partnership for Maternal Safety: Consensus Bundle on Obstetric Hemorrhage. Obstet Gynecol 2015;126:155-62. [Crossref] [PubMed]
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus Bundle on Severe Hypertension During Pregnancy and the Postpartum Period. Obstet Gynecol 2017;130:347-57. [Crossref] [PubMed]
- Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. Thousand Oaks: Sage Publications; 1994.
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago: Aldine Pub. Co. ; 1967.
- Constas MA. Qualitative analysis as a public event: The documentation of category development procedures. Am Educ Res J 1992;29:253-66. [Crossref]
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758-72. [Crossref] [PubMed]
- Strauss AL, Corbin JM. Grounded theory in practice. Thousand Oaks: Sage Publications; 1997.
- Scientific Software Development. ATLAS.ti. Berlin: Scientific Software Development; 2013.
- Council for Patient Safety in Women's Health Care. Safe Health Care for Every Woman. 2015. Available online: http://www.safehealthcareforeverywoman.org/. Accessed June 24 2016.
- Kacmar RM, Mhyre JM, Scavone BM, et al. The use of postpartum hemorrhage protocols in United States academic obstetric anesthesia units. Anesth Analg 2014;119:906-10. [Crossref] [PubMed]
- Vamos CA, Thompson EL, Cantor A, et al. Contextual factors influencing the implementation of the obstetrics hemorrhage initiative in Florida. J Perinatol 2017;37:150-6. [Crossref] [PubMed]
- Burton RA, Peters RA, Devers KJ. Perspectives on Implementing Quality Improvement Collaboratives Effectively: Qualitative Findings from the CHIPRA Quality Demonstration Grant Program. Jt Comm J Qual Patient Saf 2018;44:12-22. [Crossref] [PubMed]
- Walker DM, DePuccio MJ, Huerta TR, et al. Designing Quality Improvement Collaboratives for Dissemination: Lessons from a Multiple Case Study of the Implementation of Obstetric Emergency Safety Bundles. Jt Comm J Qual Patient Saf 2020;46:136-45. [Crossref] [PubMed]
- Pallotto EK, Chuo J, Piazza AJ, et al. Orchestrated testing: an innovative approach to a multicenter improvement collaborative. Am J Med Qual 2017;32:87-92. [Crossref] [PubMed]
Cite this article as: Walker DM, DePuccio MJ, McAlearney AS. Barriers and facilitators to hospital implementation of obstetric emergency safety bundles: a qualitative study. J Hosp Manag Health Policy 2021;5:3.

