Implications of dexamethasone and ropivacaine in peripheral nerve blocks
Introduction
Purpose
To determine the stability of ropivacaine and dexamethasone for use in peripheral nerve blockades.
The combination of ropivacaine and dexamethasone is commonly used for peripheral nerve blockades, such as transversus abdominis plane blocks, interscalene blocks, adductor canal blocks, and many other kinds of blocks. Current research suggests that this admixture crystalizes rapidly, presenting difficulty for batch preparation and storage. Current practice at our institution is to prepare the nerve blocks as needed before surgery in a non-sterile area. The pharmacy department has been contacted to possibly compound the blocks ahead of time and stock them in an automatic dispensing cabinet pending compatibility data.
After analyzing many studies on this combination of drugs, there remains a gap in the knowledge regarding the crystallization of the formulations used at this institution and peer institutions around the country. Watkins et al. demonstrated that crystallization of ropivacaine and dexamethasone occurs when used in a 1:1 combination, when using 0.75% ropivacaine and either the 10 or 4 mg/mL concentrations of dexamethasone. Though Watkins et al. demonstrated crystallization at high concentrations of dexamethasone admixed with ropivacaine, those concentrations are too dissimilar to those used at our institution for the data to be a basis for pharmacy decision-making (1). Even after calling two drug manufacturers of ropivacaine, neither could offer any further data on the compatibility of the two drugs.
Many providers at our institution have dismissed past studies on the crystallization of ropivacaine and dexamethasone because these admixtures were studied at different concentrations (i.e., much greater concentrations of dexamethasone than what is used here in clinical practice). Practitioners have also expressed anecdotally that they have never seen crystals when they mix ropivacaine with dexamethasone. It is a common misconception that if no crystals are seen macroscopically, then no crystals exist. It is also a common misconception that because no crystals are visualized, there is no risk of embolism (2). Therefore, we devised an experiment to test the crystallization of this combination of drugs in the formulations used at our institution.
With these data, we have streamlined our operating room and pharmacy practices, allowing pharmacy to prepare the nerve blocks for scheduled procedures in advance and improve time management of nurses, physicians, and pharmacists alike. Additionally, this study may enhance patient safety and provide the data necessary for other institutions to implement a more efficient method of preparing nerve blocks.
Background
Nerve blockades are used for surgical anesthesia to prevent the propagation of pain impulses along a nerve and its distal branches. Nerve blocks provide profound anesthesia of the surgical site, contributing to decreased post-operative pain and opioid use (3). For example, transversus abdominis plane blocks are commonly used for abdominal surgeries, interscalene blocks are commonly used for upper extremity surgeries, and adductor canal blocks are commonly used for surgeries such as total knee replacements. The blocks are often administered on to the nerve with the aid of an ultrasound to ensure correct placement. Prior to dispensing the medication, the needle is aspirated to ensure placement is not within the vasculature.
Nerve blockades remain an integral component of preventing complications and speeding recovery after surgeries, thereby reducing length of stay at the hospital. Adding a steroid such as dexamethasone to a local anesthetic such as ropivacaine is known to prolong the anesthetic effect, provide a faster onset of action of the anesthetic, and reduce the use of opioids post-operatively (3). Decreasing the need for post-operative pain control medications, in particular opioids, is of significant concern in modern practice. For example, post-operative opioid use increases the risk of ileus after abdominal surgeries, due to reduced intestinal motility (3). For knee surgeries, opioids may cause lethargy, nausea, or vomiting, which may stall the initiation of physical therapy, delaying recovery. Therefore, nerve blocks play a vital role in reducing opioid use post-operatively and preventing complications, allowing for a faster recovery and shorter hospital stay.
Though profound anesthesia can be achieved with plain anesthetic, various alternative formulations exist wherein other drugs like vasoconstrictors or steroids are added to confer additional benefits. Specifically, the combination of ropivacaine and dexamethasone has shown superiority in various trials over other local anesthetics as well as steroids. Ropivacaine is the anesthetic of choice at our institution for surgical blocks, due to its lower neurotoxicity and cardiotoxicity when compared other local anesthetics like bupivacaine (4,5). Dexamethasone is commonly used as it is considered a non-particulate steroid, meaning it is water-soluble, whereas other steroids like methylprednisolone and betamethasone are considered particulate steroids and should not be given intravascularly to avoid embolus. Therefore, dexamethasone is safer choice in avoiding severe complications due to embolism caused by inadvertent intravascularization upon administering a nerve block.
Though neither ropivacaine nor dexamethasone are known to crystalize and form crystalline emboli independently, in combination there is cause for concern due to the basicity of the dexamethasone solution. Ropivacaine in the presence of basic solutions is known to cause crystal formation (1). This is why many local anesthetics are prepared in the acidic range. As per the Naropin® package insert, “the solubility of ropivacaine is limited at pH above 6. Thus, care must be taken as precipitation may occur if Naropin® is mixed with alkaline solutions” (6). Though the package insert warns of precipitation, it does not specify which drugs commonly mixed with ropivacaine may cause precipitation or what ‘care’ must be taken. The crystals formed when ropivacaine is mixed with dexamethasone impose a risk of serious neurological adverse events like spinal cord injury, paralysis, and even death should they inadvertently embolize (2). It known that other local anesthetics like bupivacaine and lidocaine do not crystalize when combined with dexamethasone (7,8). However, there are other factors which do make ropivacaine an attractive anesthetic for nerve blocks which are discussed below.
Many providers underestimate the importance of ensuring that local anesthetics do not reach the vasculature. Local anesthetic systemic toxicity (LAST) is a serious consequence of anesthetic administration that is caused by either systemic absorption of local anesthetic or by accidental intravascular administration. In 2009, the incidence of LAST for peripheral nerve blockade was estimated at 9.8 per 10,000 cases (9). Though the exact frequency of LAST caused by accidental intravascular administration is unknown, it is still a high risk practice considering the potential complications should the anesthetic and crystals enter the vasculature. Providers should also be aware that ultrasound guidance of local anesthetic administration is not a foolproof method for avoiding vasculature and neither is needle aspiration. Needle aspiration may fail to identify 0.6–2.3% of needles that have been placed intravascularly (10). This produces a false negative rate that is significant and one of which providers should be aware.
Since it is known that dexamethasone alkalinizes the ropivacaine solution, causing an insoluble precipitate to form at a pH greater than 6, we tested the formulation pH and examined the extent of crystallization (or lack thereof) of the nerve block formulations used at our institution. We also examined the individual drug components in each block as a baseline. Lastly, we explored the implications of our research and the possibility of using another local anesthetic to replace ropivacaine that does not crystalize in the presence of dexamethasone.
Methods
In order to test the degree of crystallization of ropivacaine and dexamethasone, we prepared the two formulations of nerve blocks as utilized at our institution: transversus abdominis plane blocks and interscalene or adductor canal blocks. Interscalene and adductor canal blocks are prepared using the same formulation. Transversus abdominis plane blocks have a slightly different formulation with sodium chloride added. Listed in Table 1 are the formulations of the nerve blocks used at our institution, which vary in concentration from those used at other institutions. For experimentation, syringes were prepared mirroring these admixtures. We also prepared a control of each nerve block formulation. The control has sodium bicarbonate replacing the dexamethasone, since this solution would be expected to crystalize rapidly due to its highly basic nature.
Table 1
| Transverse abdominis plane block | Interscalene or adductor canal block |
|---|---|
| Two 35 mL syringes each with: | One 35 mL syringe with: |
| 22 mL of 0.5% ropivacaine | 30 mL of 0.5% ropivacaine |
| 4 mg dexamethasone (1 mL of 4 mg/mL) | 8 mg dexamethasone (2 mL of 4 mg/mL) |
| 5 mL of 0.9% sodium chloride |
For all nerve blocks prepared, we used the following drugs:
- Hospira 8.4% sodium bicarbonate injection, USP 50 mEq (1 mEq/mL in 50 mL);
- Fresenius Kabi USA 4 mg/1 mL dexamethasone sodium phosphate injection;
- APP Pharmaceuticals sodium chloride 0.9% (10 mL single dose vial);
- Fresenius Kabi USA ropivacaine 0.5%: Naropin® HCl injection (150 mg/30 mL).
Table 2 presents the admixtures we tested and the volumes of each drug used to prepare them. For purposes of simplification, we have renamed the admixtures as shown in Table 2. For example, Solution 1 represents the transversus abdominis plane block. Solution 2 represents the interscalene or adductor canal block. We also tested controls of these two admixtures. Control 1 mirrors Solution 1 but with sodium bicarbonate replacing the dexamethasone. Control 2 mirrors Solution 2 with sodium bicarbonate replacing the dexamethasone. We then observed the pH as well as the time to crystallization and the extent of crystallization.
Table 2
| Solution 1 |
| Ropivacaine (22 mL) + |
| Sodium chloride (5 mL) + |
| Dexamethasone (1 mL) |
| Solution 2 |
| Ropivacaine (30 mL) + |
| Dexamethasone (2 mL) |
| Control 1 |
| Ropivacaine (22 mL) + |
| Sodium chloride (5 mL) + |
| Sodium bicarbonate (1 mL) |
| Control 2 |
| Ropivacaine (30 mL) + |
| Sodium bicarbonate (2 mL) |
To draw up the solutions in Table 2, we used Covidien Monoject™ 35 mL polypropylene syringes with luer-lock tips. These are the same syringes that are used in surgical practice at our institution. Using pH paper (Hydrion® Papers 1–12 Micro Essential Laboratory Inc.), we estimated the pH to the nearest 0.5. Next, we examined the wet mounts of admixtures under a microscope using 20x magnification to check visually for crystals at time (t) =1 minute, 10 minutes, 60 minutes, and 120 minutes. Using the same prepared syringes which were left undisturbed at room temperature, we checked back for crystals after 24 hours. No other experiments have investigated crystallization potential out to this time point to our knowledge. We examined a fresh sample drawn from the prepared syringe on a clean slide each time. This data is presented in Table 3.
Table 3
| Admixture | pH | Crystals at t=1 minute | Crystals at t=10 minutes | Crystals at t=60 minutes | Crystals at t=120 minutes | Crystals at t=24 hours |
|---|---|---|---|---|---|---|
| Solution 1 | 6.5 | 1 | 1 | 1 | 1 | 1 |
| Control 1 | 7.5 | 5 | 5 | 5 | 5 | 5 |
| Solution 2 | 6.5 | 1 | 1 | 1 | 1 | 1 |
| Control 2 | 7.5 | 5 | 5 | 5 | 5 | 5 |
We also tested the pH and crystallization tendencies of the individual drug components utilized to prepare the nerve block admixtures and their controls, namely sodium bicarbonate, dexamethasone, sodium chloride, and ropivacaine. This information serves as a baseline to track pH changes and as a control since we know the drugs would not be expected to contain any crystals. To draw up the pure drugs, we used various BD Luer-Lok™ 1-mL, 5-mL, and 10-mL polypropylene syringes and utilized the same methods as described above for measuring the pH and checking for crystals. This baseline data is presented in Table 4.
Table 4
| Drug | pH | Crystals at t=1 minute | Crystals at t=60 minutes |
|---|---|---|---|
| Sodium bicarbonate 8.4% | 8.0 | 0 | 0 |
| Dexamethasone 4 mg/mL | 7.5 | 0 | 0 |
| Sodium chloride 0.9% | 6.5 | 0 | 0 |
| Ropivacaine 0.5% | 6 | 0 | 0 |
Results
To assess the level of crystallization in the admixtures and their individual drug components, we used an ordinal scale of 0–5 similar to Watkins et al. (1), where 0 represents no crystallization, 1 represents slight crystallization, and 5 represents substantial crystallization. The results are represented in Table 3 and Table 4.
Crystals were detected within minutes in all of the mixtures in Table 3. Control 1 and Control 2 had crystals that were visible instantaneously after mixed. These crystals were visible to the naked eye (macroscopically). However, the crystals formed in Solution 1 and Solution 2 were visible under microscopy only. By the time the solution was prepared and focused under a microscope (which took about 1 minute), crystals were present. Presumably, the crystals in Solution 1 and Solution 2 also formed near instantaneously but were not visible macroscopically.
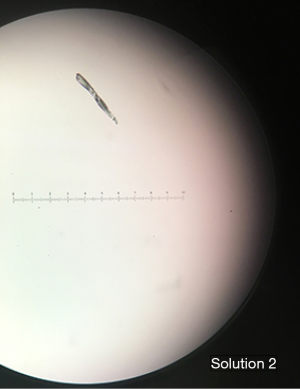
When the crystals were rechecked at a later time point, there did not seem to be a noticeable change in crystal number or size. Crystals seemed to form near instantaneously and did not appear to increase in number over time. As shown in Figure 1, most of the crystals visualized in Solution 1, Solution 2, Control 1, and Control 2 were linear. Some crystals visualized were stellate in shape. All images shown in Figure 1 are viewed via a 20× objective lens. Using an ocular micrometer, we have determined that the linear crystals in Solution 1 and Solution 2 measure on average 100–125 µm while the stellate crystals measure 50–100 µm. However, some larger crystals have been visualized. Figure 2 shows a crystal that measures upwards of 200 µm.
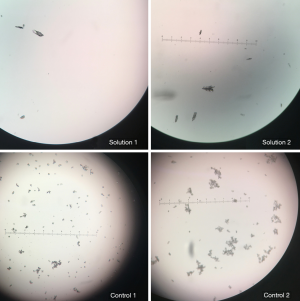
Furthermore, most of the crystals formed in Control 1 and Control 2 floated to the top of the syringe, with a minority settling at the bottom. This is shown in Figure 3 below. This pattern of crystallization likely holds true for Solution 1 and Solution 2 and may present some implications discussed later.
Discussion
Many institutions (including our own) preferentially use ropivacaine in peripheral nerve blockades for multiple reasons. First is its lower risk of cardiotoxicity in the setting of inadvertent intravascularization (1,4,6,11). The threshold for ropivacaine versus bupivacaine for causing arrhythmias and cardio-depression was much higher with ropivacaine (6). This means that more ropivacaine needs to be given before the adverse cardiac effects are seen, allowing more of a cushioning should the local anesthetic be accidentally administered intravascularly. Ropivacaine also has lower neurotoxicity when compared to bupivacaine (5). Moreover, the onset, depth and duration of the sensory block are very similar to bupivacaine but the unwanted effects of motor block are less than with bupivacaine (6). The reduced motor effects of ropivacaine are due to its lower lipophilicity, and therefore lower likelihood of penetrating large myelin fibers (11). Lastly, ropivacaine has demonstrated lower risk of chondrotoxicity when compared to bupivacaine and lidocaine (12). Unsurprisingly, many practitioners are hesitant to use bupivacaine in their practices due to the better toxicity profile of ropivacaine over other local anesthetics.
With the information gathered from our experiment, the decision to use ropivacaine should be more closely examined. First, according to the Naropin® package insert, ropivacaine’s solubility is limited at a pH greater than 6. Since dexamethasone has a pH of 7.5, its addition to acidic ropivacaine causes the pH of the mixture to increase from 6 to 6.5, even at the small concentrations in the admixtures used at this institution. Therefore, we would expect crystallization and proceed with caution when we use this admixture. Our data also suggests that the warnings associated with intravascular injection of particulate steroids may need to be extended to dexamethasone when mixed with ropivacaine due to its crystallization and risk for embolization. These risks include loss of vision, stroke, paralysis, and even death (13). It is worth noting that the FDA has expressed concern regarding the use of particulate steroids for spinal injections given the risks associated with inadvertent intravascular embolization. The FDA now recommends using non-particulate steroids to reduce these complications (13). While administering a spinal block is indisputably a more high risk procedure than administering a peripheral nerve block, it may be prudent to extend this warning of particulate steroids to other kinds of nerve blocks, considering they all have the potential to accidentally be injected intravenously.
It is also worth mentioning that a recent study in pigs assessed intravascular administration of various steroids. The particulate steroid methylprednisolone was injected into the vertebral artery of pigs and compared to pigs who received non-particulate dexamethasone via the same route. The authors concluded that all the pigs who received methylprednisolone had serious neurologic deficits and needed a ventilator while none of the pigs who received dexamethasone had any serious issues (14). While pigs and humans are physiologically different, a study like this will likely never be performed in humans given the obvious ethical issues. However, one important conclusion from this study is that non-particulate steroids had much better outcomes in the setting of inadvertent intravascularization (14).
While the risks of intravascular injection of ropivacaine and dexamethasone crystals have not been specifically studied to our knowledge, we propose that the combination should be regarded as having similar risks to infusing a particulate steroid intravascularly. There are many serious risks associated with the inadvertent intravascular exposure of a local anesthetic including neurological toxicity and cardiac arrest (15). However, it appears the risk of intravascular exposure of crystalized ropivacaine carries the additional risk of embolization, and the complications associated with it such as stroke and myocardial infarction, as well as the issues seen in pigs in the study referenced above.
For nerve blocks, using ropivacaine over another local anesthetic like bupivacaine provides the benefit of decreased cardiotoxicity, neurotoxicity, and motor blockade. However, its combination with dexamethasone provides the increased embolic risk due to the crystallization. Our data suggests that using ropivacaine is akin to trading one potential toxicity for another. It seems that using ropivacaine with dexamethasone would negate the benefits of decreased cardiotoxicity due to the added risk of inadvertently infusing crystals. Institutions now must decide which toxicity-risk is more clinically important. We suggest that institutions make it a practice to record and publish the type and rate of occurrence of complications associated with the use of ropivacaine and dexamethasone as a peripheral nerve block, as such data is largely unavailable. It would be extremely useful if the rates of morbidity and mortality associated with inadvertent intravascularization related to the cardiotoxicity and embolic risks were individually reported. However, this may be very difficult to determine. Even without this information, there is still cause for re-evaluation of anesthetic usage policies by institutions.
Impacts on current practice
We present various avenues for addressing the crystallization problem. First, we may consider using a different local anesthetic than ropivacaine provided it has a similar onset of action and duration of action. Huynh et al. conducted a meta-analysis of 12 randomized controlled trials and analyzed the combination of dexamethasone with various anesthetics for peripheral nerve blocks like transversus abdominis plane and interscalene. They found that the addition of dexamethasone approximately doubled the duration of postoperative analgesia when in combination with any of the following anesthetics: ropivacaine, lidocaine, mepivacaine, and bupivacaine (16). They also found that the addition of dexamethasone provided a significantly faster onset of action with any of the local previously mentioned anesthetics (16). Cummings et al. confirmed this data and found that the combined effect of dexamethasone and either ropivacaine or bupivacaine produced nearly the same 22 hours of analgesia for interscalene blocks. They defined duration of action as the “time from the onset of sensory block to the first use of opioid analgesia” (17).
There are mixed ideas on whether bupivacaine crystalizes. To clarify, Hwang et al. has concluded that bupivacaine has been shown to crystalize at a simulated physiologic pH using sodium hydroxide but not when actually combined with dexamethasone (18). Others have confirmed finding no crystals with bupivacaine (1,8). These studies all utilized a 1:1 concentration of local anesthetic and steroid (1,8,18), which is a much higher concentration of dexamethasone than what would be used in nerve blocks at our institution. This suggests that bupivacaine (as well as the other anesthetics mentioned above) may be used in place of ropivacaine for nerve blocks. Since only ropivacaine appears to crystalize when combined with dexamethasone, it may be worth considering another anesthetic since the onset of action and duration of action do not seem to differ significantly.
Other things to consider when choosing between ropivacaine and bupivacaine include which toxicity (embolism or cardiotoxicity) may be easier to prevent or reverse, or has less serious consequences. In comparing only the cardiotoxicity caused by ropivacaine versus bupivacaine, it appears that the cardiotoxicity caused by ropivacaine is easier to reverse. “In cases of deliberately induced cardiotoxicity, ropivacaine is associated with better reversibility by drugs and/or by electrical atrial or ventricular pacing” (4). More studies have come to the same conclusion that ropivacaine is easier to reverse after accidental intravascular injection (19). Lipid rescue is a key component of reversing LAST and should always be on hand when administering anesthetics.
If institutions are insistent that ropivacaine must continue to be used, filtering the crystals to improve the safety profile of ropivacaine may be considered. Though dexamethasone is considered a non-particulate steroid, it contains some particles that measure 0.5 µm on average (20). Theoretically, particles 0.5 µm or smaller appear safe to infuse considering that unadulterated dexamethasone contains particles of this size that may be infused. Furthermore, particles that are smaller than an erythrocyte may be thought of as safer to infuse (20). It is known that the median size of an erythrocyte is approximately 7.5 µm (21), while the crystals we observed formed in the ropivacaine and dexamethasone admixture measured much larger, some upwards of 200 µm. Larger particle sizes would be assumed to carry a higher risk for occluding a blood vessel should they enter the vasculature. This suggests that these particles do not appear safe to infuse and clearly pose a risk of embolization. Theoretically, using a filter needle of either 0.22 or 5 µm may reduce the embolic risk associated with accidentally infusing these particles.
Our data suggests that once ropivacaine and dexamethasone are mixed, crystals form very rapidly (within 1 minute), and do not seem to increase in either number or size as time goes on. This is consistent with other research (1). However, prior studies have not tracked crystals for as long as we did. We tracked the crystals for 24 hours. This suggests that if institutions decide that ropivacaine should continue to be used in peripheral nerve blocks, the ropivacaine and dexamethasone may be premixed in a syringe up to 24 hours before they are needed. However, more studies should be conducted to support this conclusion and possibly also to study the crystals out to a later time point, as the solution may or may not remain stable after the 24-hour mark that we evaluated in our studies.
Practitioners may even consider buffering the ropivacaine and dexamethasone solution. Since crystals form at a more basic pH, buffering the solution with an acid may prevent precipitate from forming. Studies have confirmed that adding a strong acid like hydrochloric acid to the solution was able to dissolve the crystals that formed (18). Additionally, temperature adjustment may be considered to dissolve the crystals. However, other studies have shown no difference in crystals when the ropivacaine and dexamethasone solution was warmed up to 40 °C (18,22). Especially in the case of temperature control, safety precautions would be required to ensure that an unmonitored temperature change does not occur, leading to the fast precipitation of crystals in the hands of the unsuspecting clinician. Another potential solution may be sequentially injecting ropivacaine and dexamethasone and not mixing them in a syringe beforehand. Additionally, drug manufacturers may also consider selling the combination in a single product, given the improved safety of industrial manufacturing controls and institutional preference for ropivacaine and dexamethasone over other combinations.
More questions need to be addressed before any of these approaches should be taken: first, the potential for particle aggregation once in the vasculature should be evaluated. Though the particles may be small enough to infuse without the risk for embolism, they may have different properties once in the vasculature, for example, depending on the rate of perfusion of the injected substance. Second, storage conditions of the premixed syringes should be further evaluated to establish the most ideal conditions. Third, the potential for recrystallization if the crystals are filtered out by various means should be studied. If the solution does recrystallize, how rapidly does this occur? Are the crystals of size to pose an embolic risk? Fourth, whether an acid buffer will alter the nerve block and what acid should be used should also be investigated. Fifth, the question of whether sequential injection of the local anesthetic and nerve block provide adequate onset and duration of action should be addressed, perhaps in combination with various local factors like injection site and perfusion rates.
Conclusions
Our experiment suggests that current anesthetic practice needs to be reassessed and altered. The ropivacaine and dexamethasone combination rapidly crystalizes even at very low concentrations of dexamethasone. Though dexamethasone is a non-particulate steroid that can be used for intravascular injection, our results suggest that the combination of the dexamethasone and ropivacaine should be regarded as a particulate steroid, and carries the same risks of intravascular administration as particulate steroids. Although there is a low potential for inadvertent intravascularization with anesthetic administration, the complications are severe if this does occur. With these data, institutions will have to decide whether there is a level (if any) of crystallization that is clinically significant or acceptable in their current protocol. Institutions must weigh the benefits of using ropivacaine versus the risks associated with inadvertent intravascularization of the crystals. One of the major benefits of ropivacaine is that it appears to be safer than bupivacaine as it has less neurotoxicity and cardiotoxicity.
Our results suggest some important limitations of our study. To elaborate, as seen in Figure 3, the crystals are less dense than the solutions they are in and float at the top of the syringe. Therefore, when collecting our samples to check for crystallization, we may have actually under-recorded how many crystals were actually present, and our samples may not necessarily reflect the full extent of the crystallization. This limitation is mitigated given that we collected all the samples in the same manner and used a subjective scale of crystallization, and therefore, all samples would be affected uniformly.
Because of the clear benefit of using ropivacaine over other local anesthetics and because the crystals seen in the ropivacaine and dexamethasone admixture do not appear to increase in either size or number with time, it may be a reasonable practice to prepare the ropivacaine and dexamethasone mixture ahead of time for the planned nerve blocks for that day. Institutions may decide to further filter the crystals to reduce the embolic risk based on a risk analysis of their protocol. There are multiple benefits to this manner of continued use of ropivacaine and dexamethasone admixtures. Pharmacy could prepare the admixture in a sterile and controlled environment, instead of in a location that may not be sterile, as is currently practiced at our institution. This also takes the compounding duty away from the anesthesiologist so that he may focus on clinical care, leaving the compounding task to the pharmacist. This improves patient safety and streamlines hospital practice by improving time and human resource management. Further research is needed to determine the compatibility, stability, recrystallization, and storage recommendations of the combination of these agents in the formulations that would be needed at this institution. Our institution has revised our protocol accordingly and will likely conduct more studies that may provide further insight into this topic.
Despite some remaining questions, the data presented in this study have several simple and actionable implications, and have been directly causative of several drug safety improvements at our institution. Many important questions have been raised regarding the safety of infusing crystalized ropivacaine and the potential for its inadvertent intravascular administration. Our pharmacy practice has been altered in a way that pays special attention to, first and foremost, improving patient safety. Though the incidence of inadvertent intravascularization of local anesthetics remains relatively low, institutions must carefully consider all of the possible solutions regarding the crystallization of ropivacaine and dexamethasone. Simple measures like those proposed here can have live-saving effects in preventing serious complications that can have a significant impact in the lives of surgery patients and their families.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp.2019.12.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Watkins TW, Dupre S, Coucher JR. Ropivacaine and dexamethasone: a potentially dangerous combination for therapeutic pain injections. J Med Imaging Radiat Oncol 2015;59:571-7. [Crossref] [PubMed]
- Kennedy DJ, Levin J, Rosenquist R, et al. Epidural steroid injections are safe and effective: multisociety letter in support of the safety and effectiveness of epidural steroid injections. Pain Med 2015;16:833-8. [Crossref] [PubMed]
- Lubawski J, Saclarides T. Postoperative ileus: strategies for reduction. Ther Clin Risk Manag 2008;4:913-7. [Crossref] [PubMed]
- Leisure GS, Di Fasio CA. Ropivacaine: the new local anesthetic. Semin Anesth 1996;15:1-9. [Crossref]
- Reiz S, Haggmark S, Johansson G, et al. Cardiotoxicity of ropivacaine a new amide local anaesthetic agent. Acta Anaesthesiol Scand 1989;33:93-8. [Crossref] [PubMed]
- Naropin [package insert]. Bad Homburg vor der Höhe, Germany: Fresenius Kabi, LLC; 2017.
- Derby R, Lee SH, Date ES, et al. Size and aggregation of corticosteroids used for epidural injections. Pain Med 2008;9:227-34. [Crossref] [PubMed]
- MacMahon PJ, Shelly MJ, Scholz D, et al. Injectable corticosteroid preparations: an embolic risk assessment by static and dynamic microscopic analysis. AJNR Am J Neuroradiol 2011;32:1830-5. [Crossref] [PubMed]
- Barrington MJ, Watts SA, Gledhill SR, et al. Preliminary results of the Australasian Regional Anaesthesia Collaboration: a prospective audit of over 7000 peripheral nerve and plexus blocks for neurological and other complications. Reg Anesth Pain Med 2009;34:534-41. [Crossref] [PubMed]
- Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth 2004;13:227-33. [Crossref] [PubMed]
- Kuthiala G, Chaudhary G. Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth 2011;55:104-10. [Crossref] [PubMed]
- Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am 2010;92:599-608. [Crossref] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. 2014. Available online: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM394286.pdf. Accessed 8 June 2017.
- Okubadejo GO, Talcott MR, Schmidt RE, et al. Perils of intravascular methylprednisolone injection into the vertebral artery: an animal study. J Bone Joint Surg Am 2008;90:1932-8. [Crossref] [PubMed]
- Bourne E, Wright C, Royse C. A review of local anesthetic cardiotoxicity and treatment with lipid emulsion. Local Reg Anesth 2010;3:11-19. [PubMed]
- Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol 2015;32:751-8. [Crossref] [PubMed]
- Cummings KC, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine Br J Anaesth 2011;107:446-53. [Crossref] [PubMed]
- Hwang H, Park J, Lee WK, et al. Crystallization of local anesthetics when mixed with corticosteroid solutions. Ann Rehabil Med 2016;40:21. [Crossref] [PubMed]
- Stienstra R. The place of ropivacaine in anesthesia. Acta Anaesthesiol Belg 2003;54:141-8. [PubMed]
- Dietrich TJ, Sutter R, Frolich J, et al. Particulate versus non-particulate steroids for lumbar transforaminal or interlaminar epidural steroid injections: an update. Skeletal Radiol 2015;44:149-55. [Crossref] [PubMed]
- Yin W, Bogduk N. Retrograde filling of a thoracic spinal artery during transforaminal injection. Pain Med 2009;10:689-92. [Crossref] [PubMed]
- Koitabashi T, Sekiguchi H, Miyao H, et al. Precipitation of pH-adjusted local anesthetics with sodium bicarbonate. Masui 1995;44:15-20. [PubMed]
Cite this article as: Tow BA. Implications of dexamethasone and ropivacaine in peripheral nerve blocks. J Hosp Manag Health Policy 2020;4:4.