
Enabling rapid intervention and isolation for patients with highly infectious diseases at points of need
Assessing interventions for Ebola
The 2014–2015 Ebola virus disease (EVD) epidemic in West Africa proved unequivocally the need for immediate intervention to control disease transmission. The efforts of the World Health Organization (WHO), Center for Disease Control and Prevention (CDC), United Nations (UN), UNISEF, United States Agency for International Development (USAID) and Liberian Ministry of Health and Social Welfare (MOHSW), while initially slow to respond, helped quell the EVD epidemic.
In 2016, Kirsch and colleagues reported no single interventional effort stopped the 2014–2015 EVD epidemic in Liberia. Rather, the several interventional processes produced a reinforcing effect (1). The authors present a systematic framework of analysis comparing a timeline of government and international activities by the UN, CDC, WHO, UNICEF, USAID, and MOHSW versus an epidemic curve. An epidemic curve visualizes graphically the estimated efficiency of EVD transmission (Rt ; reproductive number), as a trajectory of the epidemic in Liberia (Rt >1; growing epidemic, Rt <1; diminishing epidemic) with relation to time (key activities).
Kirsch and colleagues explored the initiation of response activities (e.g., creating laboratories, isolating infected individuals, building Ebola treatment units, supplying personal protective equipment, performing proper burials, contact tracing, and community outreach) to completion and their effects on modulating the shape of the epidemic curve (shown in their Figure 1). However, before any intervention was fully implemented, the epidemic curve diminished. Each intervention strengthened another to reduce the size of the epidemic.
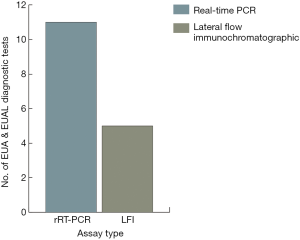
The observations of Kirsch et al. (1) do not suggest a solution other than transiently enacted measures by the U.S. and other nations to enhance community resilience for EVD and for other threats. They do not focus on decision making or outcomes, per se, and wisely end the article short without implying the needs for diagnostic testing at points of need. According to the WHO, “The goal of interrupting chains of Ebola virus transmission depends heavily on laboratory support. This support is needed to confirm or discard suspected cases, guide triage and clinical decisions, aid contact tracing, and facilitate the early detection of cases…and isolation likewise depends on laboratory support” (2). Chertow et al. found that in a resource-limited area managing 700 Ebola patients in Monrovia, Liberia, clinical decision making guided by rapid point-of-care (POC) diagnostic test results could significantly improve patient health outcomes (3). Hence, placement of POC tests at points of need will enable timely community surveillance, patient isolation, and targeted treatment therapy.
Designing Spatial Care PathsTM in healthcare small-world networks
We define a Spatial Care PathTM as the most efficient route for a patient to receive definitive care in a small-world network (SWN). Point-of-care technologies at points of need (e.g., homes, ambulances, and primary care settings) can facilitate timely evidence-based decision making. In a study by Cooper et al. the EVD epidemic led to the breakdown of essential health services for endemic infectious diseases and non-communicable diseases in primary care settings, forcing people to seek care at Ebola treatment centers (4). This places vulnerable groups at risk of being exposed and contracting EVD at such facilities (5). During the initial clinical phase, patients with EVD can be difficult to distinguish from patients with other infectious diseases, including malaria, typhoid fever, Lassa fever, and measles (6-8). In a study by Moses et al. fewer than half of all confirmed malaria cases received treatment during the EVD epidemics (7). A study by Truelove et al. suggests that inexpensive POC tests would be useful in differentiating EVD versus measles in patients in low-resource settings where there is limited access to laboratory testing (5). Early diagnosis and quarantine during an outbreak abate EVD transmission and build trust between community members and health services (8-10).
The transportation of people traveling to Ebola health facilities for care enabled a rapid and extensive spread of EVD in Sierra Leone (11). Kirsch and colleagues, report the second wave of the EVD epidemic in Liberia began with patients from remote villages that traveled to the urban center (1). In contrast, the EVD outbreak in the Democratic Republic of Congo was smaller in size due to its remote isolation, but more difficult to control because it took place in a war zone off limits to aid workers (11). Placement of POC tests upstream the SCP at the point of need where the first contact with a sentinel case patient is made can prevent sick febrile patients from spreading the disease to others downstream in tertiary care facilities (12). Based on POC test results, the patient can progress through the SWN of healthcare to quarantine (13) and also identify the proper loci for ring vaccination.
Diagnostic testing for Ebola
Table 1 presents EVD diagnostic tests authorized for emergency use by the FDA and WHO in descending order from newest to oldest with their respective method, specimen type, and time to results. Real-Time RT-PCR (rRT-PCR) testing is accurate and has become one of the gold standards for EVD diagnosis (see Figure 1). However, until miniaturized technologies are perfected for field use, diagnosis by rRT-PCR requires laboratory infrastructure, operation and maintenance, and personnel with expertise in molecular techniques.
Table 1
| Instrument(s) and/or assay/Kit (Manufacturer) | Principle | Samples(s) | Time to results (hours) | WHO/FDA EUAa,b |
|---|---|---|---|---|
| DPP Ebola Antigen System (Chembio Diagnostic Systems, Inc.) | LFI assay | Fingerstick WB, venous WB, plasma | Est. 0.25 | FDA 11/09/18 [not for screening, e.g., in airports or contact tracing] |
| Idylla Ebola Virus Triage Test (Biocartis NV) | rRT-PCR | venous WB | 1.67 | FDA 5/26/16 |
| SD Q Line Ebola Zaire Ag (SD Biosensor Inc.) | LFI assay | WB, plasma, serum | 0.33–0.5 | WHO August 2015 |
| OraQuick Ebola Rapid Antigen Test (OraSure Technologies, Inc.) | LFI assay | Venous WB & fingerstick WB; cadaveric oral fluid | 0.5 | WHO & FDA 7/31/15, 3/04/16 [not for screening, e.g., in airports or contact tracing] |
| Liferiver Ebola virus (EBOV) Real-Time RT-PCR Kit (Shanghai ZJ BioTech) | rRT-PCR | WB, plasma, serum | 4-6 | WHO April 2015 |
| Xpert Ebola Assay (Cepheid) | Automated cartridge-based rRT-PCR | FDA: WB (venipuncture, fingerstick, oral swab) WHO: venous WB | 1.63 | WHO & FDA 3/23/15 |
| ReEBOV Antigen Rapid Test Kit (Corgenix) | LFI assay | plasma, serum | 0.25–0.42 | WHO February 2015 |
| LightMix Ebola Zaire rRT-PCR Test for use with LightCycler 480 or cobas z 480 (Roche Molecular Systems, Inc.) | rRT-PCR | WB or TriPure-inactivated WB | >3 | FDA 12/23/14 |
| RealStar Filovirus Screen RT-PCR Kit 1.0 (altona Diagnostics GmbH) for use with ABI Prism 7500 SDS and 7500 Fast SDS (Applied Biosystems), LightCycler 480 Instrument II (Roche), CFX96TM system/Dx Real-Time system (BIO-RAD), Mx 3005P QPCR (Stratagene), Rotor-Gene 3000/6000 (Corbett Research), Rotor-Gene Q 5/6 plex (Qiagen), Versant kPCR Molecular System AD (Siemens) | rRT-PCR | Plasma | 2 (positive result); |
WHO 11/25/14 |
| RealStar Ebolavirus RT-PCR Kit 1.0 (altona Diagnostics GmbH) for use with ABI Prism 7500 SDS and 7500 Fast SDS (Applied Biosystems), LightCycler 480 Instrument II (Roche), CFX96TM system/Dx Real-Time system (BIO-RAD) | rRT-PCR | Plasma | Varies with instrument | FDA 11/10/14 (RI: 11/26/15) |
| FilmArray NGDS BT-E Assay (BioFire Defense, LLC) | Automated cartridge-based rRT-PCR | WB, plasma, serum | 1 | FDA 10/25/14 (RI:3/2/15) |
| FilmArray BioThreat-E test (BioFire Defense, LLC) | Automated cartridge-based rRT-PCR | WB, urine (if matched to blood) | 1 | WHO & FDA 10/25/14 |
| CDC Ebola Virus NP & VP40 Real-Time RT-PCR Assays (CDC) for use with 7500 Fast Dx Real-Time PCR (Applied Biosystems) & CFX96 Touch Real-Time PCR (BIO-RAD) | rRT-PCR | WB, plasma, serum, urine (if matched to blood) | NS | FDA 10/10/14 (RI:3/02/15) |
| EZ1 rRT-PCR Assay (DoD) for use with 7500 Fast Dx (Applied Biosystems), LightCycler (Roche), & JBAIDS (BioFire Defense) | rRT-PCR | Trizol-inactivated WB & plasma | Varies with |
FDA 08/05/14 (RI:10/10/14) |
CDC, centers for disease control and prevention; DOD, department of defense; EUA, emergency use authorization; EZV, Ebola zaire virus; FDA, food and drug administration; LFI, lateral flow immunochromatographic assays; NS, not specified in the EUA; RI, reissued by the FDA on the given date; rRT-PCR, real-time reverse transcriptase polymerase chain reaction; WB, whole blood; WHO, World health organization. a, U.S. FDA Emergency Use Authorization status can be found at
Novel pathogen identification diagnostic platforms, such as automated nucleic acid tests (NATs) and rapid antigen detection tests (RDT) successfully integrated into testing algorithms, could have an immediate impact at points of need in decentralized health care settings with minimal laboratory infrastructure (14,15). If sensitivity [TP/(TP + FN)] is low, then FN is high and false negative (FN) individuals can transmit EVD, contributing to the growth of an outbreak. However, POC RDT still confers substantial benefits as public health resources in limited remote settings by improving case management and infection control measures (14).
Ruling out and ruling in with a diagnostic test
Ruling out a highly infectious disease requires high sensitivity, while ruling in the diagnosis requires high specificity. The ideal POC test has both. Note also that FN = FN(t), that is, FN is a function of time. Initially, say during the first 72 hrs, FN(t) may be high because the target of the diagnostic test is not easily detectable. For this reason, we caution readers that if symptoms and signs persist, a patient deemed initially not to have EVD should be retested at 72 hrs to reduce the risk of a missed diagnosis and propagation of the outbreak. Of course, the post hoc EBM metric, negative predictive value [NPV = TN/(TN + FN(t))] will reflect the same sort of temporal weakness initially. Physicians on site tend to think and act in terms of positive predictive value [PPV = TP/(TP + FP)] and NPV. Therefore, POC tests for EVD should be evaluated in a manner that ensures both high PPV and high NPV under field use conditions, which is very challenging, if not impossible, to demonstrate currently.
Assessing current technologies
Automated cartridge-based NATs, such as the GeneXpert and FilmArray platforms provide rapid results with minimal training necessary. However, they require careful consideration of biosafety and operational challenges (e.g., extreme environmental conditions, quality control, and access to uninterrupted electricity) (12,14,15). More recently, surface-enhanced Raman scattering (SERS) technology has been used to detect Ebola, malaria, and Lassa fever infections (16). The SERS assay can be useful because of its minimal steps, lessened biosecurity concerns, environmental robustness, portability, and reasonable time to result (~30 minutes). The SERS technology has the ability to multiplex and can identify co-infections that can potentially complicate clinical treatment decisions (16). A study by Zeng et al. utilized a paper-based SERS chip on a smartphone that served as a miniaturized Raman spectral analyzer at the POC (17).
Key features of lateral flow immunoassays (LFI) include simplicity and low cost, which make them appealing for widespread use in austere environments. These tests are suitable for personnel with varying levels of training (18). According to Boisen et al., LFI diagnostic tests are environmentally robust and can withstand a wide range of temperature conditions, making them suitable for harsh regions (18). More recently, a novel use of recombinase polymerase amplification (RPA) in lateral flow strips is being used to detect EVD. This assay takes approximately 30 minutes to produce results; components cost about USD $10, which is useful in low-resource settings (19). Recombinase polymerase amplification tests generate results rapidly (~20 minutes) and use smaller reagents and equipment, while yielding high analytical sensitivity and specificity similar to that of rRT-PCR (20). A study by Yang et al. reported RPA testing performance was unaffected under simulated environmental conditions. Hence, RPA enjoys significant advantages for POCT, particularly emerging infectious diseases (20).
Enabling community resilience
In community settings, where pathogen-specific identification diagnostic tests are unavailable, POC hematology tests results may be useful in clinical decision making. The Ebola virus induces massive lymphocyte apoptosis, and thus, changes in POC hematology test results, such as white blood cell (WBC) and differential (e.g., neutrophil and lymphocyte) counts, when decreased at points of need, can signal early signs of infection, resulting in actions that help abate outbreaks from developing further (12,13,15).
Assessing suspected EVD cases in primary care upstream settings avoids the risks associated with infected patients transmitting to others downstream in the SWN and also helps avoid propagating specimens that can contaminate hospital laboratory services (21). Early detection of EVD patients with proper biohazard precautions can enable timely isolation, and the potential for introducing early antiviral therapy well before clinical symptoms appear (9,12).
Isolation facilities and isolators are fundamental to preventing the spread of highly infectious disease threats, treating critically ill patients with highly infectious diseases, and averting contamination of laboratory services (12,15). Wong et al. concluded that the Severe Acute Respiratory Syndrome (SARS) epidemic in Hong Kong, China, in 2003, was exacerbated by the lack of POC molecular diagnostics and proper isolation facilities for infected patients. Contagion escalated the rampant spread among patients and healthcare workers (22). Hence, isolation facilities equipped with isolators or biohazard cabinets for the safe workup of specimens from patients suspected to have highly infectious diseases, can assist in rapid diagnosis and guided therapy at points of need.
Self-monitoring via FAST•POCTM
We define facilitated-access self-testing point-of-care (FAST•POCTM) as the patient obtaining his or her own sample (capillary) with a self-contained sampling device (e.g., retractable lancelet, microcassette, microcuvette, or cartridge) that seals for automated testing on a POC instrument, while a “facilitator,” instructs and guides with limited or no exposure to infectious disease agents (12). Detecting EVD early and upstream in the SCP, even in homes (using FAST•POCTM), primary care sites, and emergency rooms will prevent infected patients from disseminating the disease throughout the community, in particular, to vulnerable groups (12,15). Directly performing POC testing at points of need in community settings can enhance community resilience for outbreaks and avoid circuitous referrals to distant regional laboratories (12,13,15).
Shifting the care paradigm nearer patient homes
The 2014–2015 EVD outbreak was associated with inadequate public health preparedness at points of need. Sustainable investments are needed to improve the capacity of primary health settings in health systems worldwide, before the next epidemic or pandemic strikes (5,15,23). Wagenaar et al. suggests that the effects of future EVD outbreaks on primary healthcare will exceed the direct effects from EVD infection, resulting in slow economic growth (9,24). Thus, national policies and guidelines should focus on improving primary healthcare settings by implementing POCT upstream the SCP near patient homes to assist in identifying infected individuals in need of isolation. National POCT policy can also help assure that preventative measures are funded properly by government agencies. Access to easy, low-cost POC technologies can enable community health workers and patients to better understand EVD and make effective decisions regarding disease management.
Conclusions
- National policies and guidelines should focus on steps necessary to enable future public health preparedness by immersing public health practitioners in POCT education and training.
- Governments should strategize an essential diagnostics list, especially for tests that can be delivered at points of need. Point-of-care molecular diagnostics should be supported financially in order to quickly detect and stop highly infectious disease outbreaks (see Figure 2), and to support critically ill patients placed in isolation (15).
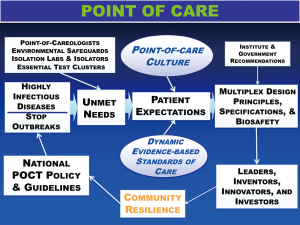 Figure 2 Framework for POCT—policy and guidelines will stop highly infectious disease outbreaks. This framework is needs-based, adaptable, and dynamic with feedback. Coordinated and integrated development and implementation of molecular diagnostics at points of need promotes rapid response to stop outbreaks. Future sustainability seems unlikely without key elements and personnel in place, specifically Point-of-Careologists, a new specialty in China, all supported by national POCT policy and guidelines. Expectations unite POC culture and standards of care to motivate the right design principles, specifications, and biosafety.
Figure 2 Framework for POCT—policy and guidelines will stop highly infectious disease outbreaks. This framework is needs-based, adaptable, and dynamic with feedback. Coordinated and integrated development and implementation of molecular diagnostics at points of need promotes rapid response to stop outbreaks. Future sustainability seems unlikely without key elements and personnel in place, specifically Point-of-Careologists, a new specialty in China, all supported by national POCT policy and guidelines. Expectations unite POC culture and standards of care to motivate the right design principles, specifications, and biosafety. - Accessible indirect screening tests (e.g., WBC and lymphocyte count) may provide early clues of infection (12).
- Isolation laboratories should be equipped with biosafety cabinets or isolators used for the safe operation of molecular diagnostics and POC technologies (15).
- Point-of-care testing embedded upstream in the SCP near patient homes, in primary care settings, and onboard ambulances would allow timely diagnosis and accelerated clinical decision making for patients with highly infectious diseases (23), rather than depending on distant reference laboratories (12).
- National POCT policies and guidelines should integrate ring vaccination, for example, using the rVSV-ZEBOV vaccine, which offers optimal health outcomes to contacts of patients with EVD (25).
Recommendations
- We strongly recommend the development of a rapid POC Ebola tests multiplexed with malaria, typhoid fever, Lassa fever, and measles to bring dynamic evidence-based decision making to points of need (7,18).
- Sustainable investments in primary healthcare can strengthen and help build community resilience capable of mitigating outbreaks before they emerge into the next epidemic or pandemic (24).
Acknowledgments
The authors appreciate the contribution of Adam Bonislawski to Figure 1. Table and figures are provided courtesy and permission of Knowledge OptimizationTM, Davis, California.
Funding: This work was supported by the Point-of-Care Testing Center for Teaching and ResearchTM (POCT•CTRTM).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp.2018.12.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirsch TD, Moseson H, Massaquoi M, et al. Impact of interventions and the incidence of ebola virus disease in Liberia—implications for future epidemics. Health Policy Plan 2017;32:205-14. [Crossref] [PubMed]
- WHO. Urgently needed: rapid, sensitive, safe and simple Ebola diagnostic tests. Ebola situation assessment [published 18 November 2014, accessed 19 December 2018]. Available online: https://www.who.int/mediacentre/news/ebola/18-november-2014-diagnostics/en/
- Chertow DS, Kleine C, Edwards JK, et al. Ebola Virus Disease in West Africa — clinical manifestations and management. N Engl J Med 2014;371:2054-7. [Crossref] [PubMed]
- Cooper C, Fisher D, Gupta N, et al. Infection prevention and control of the Ebola outbreak in Liberia, 2014–2015: key challenges and successes. BMC Med 2016;14:2. [Crossref] [PubMed]
- Truelove SA, Moss WJ, Lessler J. Mitigating measles outbreaks in West Africa post-Ebola. Expert Rev Anti Infect Ther 2015;13:1299-301. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Morbidity and Mortality Weekly Report (MMWR). [published 17 July 2017, accessed 19 December 2018]. Available online: https://www.cdc.gov/mmwr/volumes/65/su/su6503a2.htm
- Moses FL, Tamang D, Denisiuk O, et al. Management of malaria in children with fever in rural Sierra Leone in relation to the 2014–2015 Ebola outbreak. Public Health Action 2017;7:S22-S26. [Crossref] [PubMed]
- Scott V, Crawford-Browne S, Sanders D. Critiquing the response to the Ebola epidemic through a Primary Health Care Approach. BMC Public Health 2016;16:410. [Crossref] [PubMed]
- Wojda TR, Valenza PL, Cornejo K, et al. The Ebola Outbreak of 2014-2015: From Coordinated Multilateral Action to Effective Disease Containment, Vaccine Development, and Beyond. J Glob Infect Dis 2015;7:127-38. [Crossref] [PubMed]
- Kost GJ, Zhou Y, Katip P. Understanding of point-of-care culture improves resilience and standards of care in limited-resource countries. In: Kost GJ, Curtis CM, editors. Global point of care: strategies for disasters, emergencies, and public health resilience. Washington, DC: AACC Press-Elsevier; 2015:471-90, Chapter 43.
- Lu HJ, Qian J, Kargbo D, et al. Ebola Virus Outbreak Investigation, Sierra Leone, September 28–November 11, 2014. Emerg Infect Dis 2015;21:1921-7. [Crossref] [PubMed]
- Kost GJ, Ferguson W, Truong AT, et al. Molecular detection and point-of-care testing in Ebola virus disease and other threats: a new global public health framework to stop outbreaks. Expert Rev Mol Diagn 2015;15:1245-59. [Crossref] [PubMed]
- Kost GJ, Ferguson W, Hoe J, et al. The Ebola Spatial Care PathTM: Accelerating point-of-care diagnosis, decision making, and community resilience in outbreaks. Am J Disaster Med 2015;10:121-43. [Crossref] [PubMed]
- Broadhurst MJ, Brooks TJ, Pollock NR. Diagnosis of Ebola Virus Disease: past, present, and future. Clin Microbiol Rev 2016;29:773-93. [Crossref] [PubMed]
- Kost GJ. Molecular and point-of-care diagnostics for Ebola and new threats: National POCT policy and guidelines will stop epidemics. Expert Rev Mol Diagn 2018;18:657-73. [Crossref] [PubMed]
- Sebba D, Lastovich AG, Kuroda M, et al. A point-of-care diagnostic for differentiating Ebola from endemic febrile diseases. Sci Transl Med 2018;10:eaat0944.
- Zeng F, Mou T, Zhang C, et al. Paper-based SERS analysis with smartphones as Raman spectral analyzers. Analyst 2018;144:137-42. [Crossref] [PubMed]
- Boisen ML, Oottamasathien D, Jones AB, et al. Development of Prototype Filovirus Recombinant Antigen Immunoassays. J Infect Dis 2015;212:S359-67. [Crossref] [PubMed]
- James AS, Todd S, Pollak NM, et al. Ebolavirus diagnosis made simple, comparable and faster than molecular detection methods: preparing for the future. Virol J 2018;15:75. [Crossref] [PubMed]
- Yang M, Ke Y, Wang X, et al. Development and evaluation of a rapid and sensitive EBOV-RPA test for rapid diagnosis of Ebola Virus Disease. Sci Rep 2016;6:26943. [Crossref] [PubMed]
- Gilbert GL. Laboratory testing in management of patients with suspected Ebolavirus disease: infection control and safety. Pathology 2015;47:400-2. [Crossref] [PubMed]
- Wong ATY, Chen H, Liu SH, et al. From SARS to Avian Influenza preparedness in Hong Kong. Clin Infect Dis 2017;64:S98-104. [Crossref] [PubMed]
- Kost GJ, Zadran A, Thuan DTB, et al. Point-of-Care Diagnosis of Acute Myocardial Infarction in Central Vietnam: International Exchange, Needs Assessment, and Spatial Care Paths. Point Care 2018;17:73-92. [Crossref] [PubMed]
- Wagenaar BH, Augusto O, Beste J, et al. The 2014–2015 Ebola virus disease outbreak and primary healthcare delivery in Liberia: time-series analyses for 2010–2016. PLoS Med 2018;15:e1002508 [Crossref] [PubMed]
- Piot P, Spencer J. From 1976 to 2018: reflections on early investigations into the Ebola virus. Trans R Soc Trop Med Hyg 2018;112:527-8. [Crossref] [PubMed]
Cite this article as: Zadran A, Kost GJ. Enabling rapid intervention and isolation for patients with highly infectious diseases at points of need. J Hosp Manag Health Policy 2019;3:4.


