The evidence for using mHealth technologies for diabetes management in low- and middle-income countries
Introduction
Both high-income countries (HICs) and low- and middle-income countries (LMICs) are grappling with the challenges of effectively managing escalating diabetes epidemics (1,2). Annually, 4.6 million deaths worldwide are directly attributable to diabetes, 80% of which occur in LMICs (3-6). Most people with diabetes worldwide do not meet International Diabetes Federation (IDF) treatment targets of glycaemic control, i.e., glycosylated haemoglobin (HbA1c) ≤7% (2,7-10). The proportion of people with diabetes achieving this target is lowest in LMICs (1,11,12). LMICs report younger average age of diabetes onset, higher rates of diabetic complications and mortality and sharper increases in prevalence rates than HICs (3,4,6).
In many LMICs, diabetes care is compromised by many patient-related, societal and health system factors (1). Patient-related factors include poor knowledge of the disease and its treatment (1,4). Societal and health system factors include diabetes workforce shortages, lack of standardized care protocols, inadequate infrastructure and unaffordability due to poverty and limited public funding (1,4,11,13). There is an urgent need for cost-effective and widely accessible strategies for empowering and motivating people with diabetes to adhere to best-practice diabetes self-care behaviours (12,14-17). The expanding information and communication technologies (ICT) industry has received considerable interest for its potential to assist with the worldwide failure to control diabetes (18,19).
Mobile health (mHealth), has been defined as the use of mobile communication devices to transmit information with the objective of advancing health (19,20). In low-income countries (LICs), mobile communication technology is the most rapidly growing sector of the ICT industry, and geographical coverage even in these economies is high (21). As of 2015, an estimated 80% of the world’s population possessed a mobile device (20). These technologies have been proposed as cost-effective tools to supplement clinician visits and means to deliver continuity of care, which could overcome the clinician shortages particularly evident in LMICs (5,14,19,22).
mHealth technologies possess a variety of attributes that may enable them to deliver benefits to healthcare consumers and healthcare providers (18,20). mHealth tools can facilitate real-time communication between healthcare providers and patients (18,20). They can provide timely, convenient, high-quality and personalized support (14,18,19,23). The bi-directional exchange of information enabled by mobile devices means patients can be effectively monitored from a distance (20).
There have been numerous systematic reviews of mHealth applications for diabetes management, many of which have reported positive intervention effects (14,21,24-27). However, others have been less conclusive (9,28,29). While there is growing evidence that various mHealth devices and applications have the potential to improve clinical and/or behavioural diabetes-related outcomes, all these reviews exclusively or predominantly included studies conducted in HICs. Consequently, the evidence to support the use of mHealth interventions for diabetes care in LMICs is less clear (22).
Critical differences in mobile phone usage between HICs and LMICs preclude the extrapolation of findings from HICs to LMICs (18,30). Furthermore, mHealth interventions have the potential to be widespread and offer great benefit in these regions if found to be effective (18,31). There have been numerous recently reported trials of mHealth interventions for diabetes management in LMICs. However, a formal review of this nascent, yet rapidly flourishing field is yet to be conducted. To address this research gap, the present review synthesizes the evidence in this field to the present time, identifies gaps in the research, and offers directions for future research.
Methods
Search strategy
Electronic searches of PubMed, Ovid Medline, CINAHL and SCOPUS databases were conducted, seeking eligible studies published in English in a peer-reviewed journal between September 2000 and December 2017 (inclusive) and available in full text. The search strategy comprised three categories of terms: ‘diabetes’, ‘mobile health technology’ and ‘low- and middle-income countries’ and synonyms of each.
Study selection process
One reviewer screened the titles and abstracts of all search results based on the inclusion and exclusion criteria. The full texts of all potentially eligible studies were retrieved. The same reviewer screened all full texts and assessed each study for its eligibility. In cases of uncertainty, a second reviewer assessed the study for eligibility.
Inclusion criteria
To be included in this review, studies must have trialled interventions using mobile devices with the capacity for mobile and/or wireless communication and/or devices with software applications. Included studies must have trialled interventions that incorporated a two-way flow of information. That is, interventions must have been interactive insofar as the information/recommendations communicated to the user was personalized and dependent on the information provided by that person. To be included, studies must have trialled an intervention where the mHealth component was the key feature of the intervention. To be included, interventions could have been directed at any level of care, i.e. targeted directly at patients or at healthcare providers to improve the advice or care given to patients. Additionally, interventions must have been designed to improve key diabetes outcomes, either self-care behaviours (e.g., medication adherence) or clinical measures (e.g., HbA1c). Studies must have evaluated the intervention on: at least one diabetes-related clinical outcome measure; at least one diabete self-care behaviour; or, hospital admissions, or mortality.
Included studies must have described a randomized controlled trial (RCT), a controlled trial with non-random allocation, a randomized head-to-head trial, or a systematic review with meta-analysis that included only primary studies of these designs. These study designs were considered the most rigorous for assessing intervention effects.
Inclusion criteria for participants were: studies conducted in any LMIC according to World Bank classification data as of 2018; and participants who were patients must have had diabetes mellitus of any type. The year 2018 was chosen as countries tend to move up rather than down in income classification and is therefore the most restrictive criterion for income level (32).
Assessing risk of bias in included studies
The Cochrane Collaboration’s tool for assessing risk of bias (33) was utilized for included studies. The primary reviewer assessed each study on each criterion of this tool and consulted a second reviewer in cases of uncertainty. This tool consists of two criteria for assessing selection bias and one criterion each for assessing performance/detection bias, attrition bias, reporting bias and other sources of bias.
Results
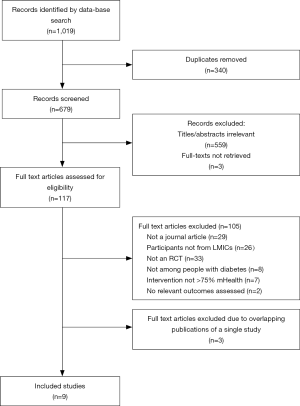
The search strategy identified 1,019 results. After removing duplicate records, there were 679 unique records. Of these, 559 were excluded after reviewing the title and abstract. Of the remaining 120 records, three of the full texts were not retrieved within the timeframe despite numerous attempts and were thus excluded. Based on a full text review of the remaining 117 records, 105 results were excluded, as they did not satisfy all the inclusion criteria. Twelve results met the inclusion criteria but three were duplicate publications of a single study. Two such publications were excluded from the present review, as both have subsequently been retracted by the respective publishers. The third was excluded because it was a summary of the other articles and lacked sufficient detail to determine eligibility. Thus, nine studies were included in the final analysis. Figure 1 represents the results of the database search and study selection process.
Characteristics of included studies
The characteristics of included studies are depicted in Table 1.
Table 1
| Author, year (ref) | Country | Study design | Sample size (baseline/follow-up) | Age range (years) | Type of diabetes | Gender (male %) | Study duration (months) | mHealth intervention (+ additional components) | Comparator condition | Outcomes measured | Outcomes tested as between-group analyses |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moattari et al., 2013 (34) | Iran | RCT | 52/48 | 18–39 | Mostly type 1 | 43 | 3 | Online platform containing personalized educational content and portal where patients entered daily data and received weekly feedback from physicians. Users could ask questions into the portal and receive answers from healthcare providers. Also involved chatroom with other users | Education advice as usual | HbA1c, fasting blood sugar, total cholesterol, triglycerides, HDL, LDL | HbA1c, fasting blood sugar, total cholesterol, triglycerides, HDL, LDL |
| Takenga et al., 2014 (35) | The Democratic Republic of Congo | RCT | 40/31 | 35–75 | Type 2 | 72.5 | 2 | Mobil Diab: Smartphone or web-based portal where patients uploaded data and received feedback (incl. therapy plans) from doctors | Treatment as usual | Blood glucose, amplitude of glycaemic excursions, HbA1c | Nil |
| SMS alerts were sent to doctors in emergency cases | |||||||||||
| Zhou et al., 2014 (36) | China | RCT | 108/108 | 18–75 | Type 2 | Unspecified | 3 | Online portal where patients entered clinical and behavioural diabetes-related data at least once/fortnight. Personalized feedback received from staff through the portal, email, SMS and phone call | Treatment as usual + educational session | BMI, systolic and diastolic blood pressure, fasting blood sugar, HbA1c, total cholesterol, triglycerides, HDL, LDL, hypoglycemic episodes | BMI, systolic and diastolic blood pressure, fasting blood sugar, HbA1c, total cholesterol, triglycerides, HDL, LDL, hypoglycemic episodes |
| Participants also attended educational session (same as control) | |||||||||||
| Kaur et al., 2015 (37) | India | RCT (3 arms) | 120/120 | >18 | Type 1 or Type 2 | 51 | 3 | Weekly telephone consultations between patient and provider to review weekly glucose testing results | Out-patient visits | HbA1c, total cholesterol, HDL, LDL, triglycerides, fasting blood sugar, PPBS, adherence to medication, adherence to diet and exercise advice, emergency admissions | Fasting blood sugar, PPBS, HbA1c |
| Participants also attended out-patient visits | |||||||||||
| Lee et al., 2015 (38) | Malaysia | RCT | 37/32 | 18–75 | Type 2 | 43 | 1.5 | Online portal that transmitted patient data to providers. Data was obtained via web-enabled glucometer, which participants were asked to use 5 times/day. Patients received feedback following concerning glucose readings | Treatment as usual | Hypoglycemic episodes, fructosamine, total cholesterol, triglycerides, HDL, LDL, fasting plasma glucose | Hypoglycemic episodes, fructosamine, total cholesterol, triglycerides, HDL, LDL, fasting plasma glucose |
| Shahid et al., 2015 (39) | Pakistan | RCT | 440/440 | 18–70 | Type 2 | 61.4 | 4 | Fortnightly telephone consultations between patient and provider. During consultations, patients relayed their BG recordings and self-care behaviours over the preceding fortnight and received immediate feedback from the provider. Intervention participants were provided the same educational material and services as control patients | Treatment as usual + educational material | Hypertension, systolic and diastolic blood pressure, BMI, LDL, HbA1c, diet, physical activity | HbA1c |
| Anzaldo-Campos et al., 2016 (40) | Mexico | RCT (3 arms) | 201/201 | 18–75 | Type 2 | 31 | 10 | MyGlucoHealth: patients entered diabetes tracking data to an online portal via a web-enabled glucometer and electronic surveys. Providers viewed the portal and provided feedback to patients on their clinical and behavioural recordings. Providers were alerted to concerning readings | Care management plan delivered by multi-disciplinary team | HbA1c, total cholesterol, HDL, LDL, triglycerides, systolic and diastolic blood pressure, BMI, diabetes self-care behaviours | HbA1c, total cholesterol, HDL, LDL, triglycerides, systolic and diastolic blood pressure, BMI, diabetes self-care behaviours |
| Participants also received a care management plan by a multi-disciplinary team (same as control) | |||||||||||
| Zhou et al., 2016 (41) | China | RCT | 100/100 | 18–74 | Type 1 or Type 2 | 57 | 3 | Welltang smartphone app: patients uploaded self-care data and providers viewed the data and relayed personalized feedback (incl. medication regimes). Alerts sent to both patients and providers in instances of concerning readings | Treatment as usual | HbA1c, fasting blood glucose, postprandial blood glucose, LDL, weight, BMI, waist and hip circumference, systolic and diastolic blood pressure, self-care behaviours, hypoglycemic events | HbA1c, fasting blood glucose, postprandial blood glucose, LDL, weight, BMI, waist and hip circumference, systolic and diastolic blood pressure, self-care behaviours, hypoglycemic events |
| Kleinman et al., 2017 (42) | India | RCT | 90/80 | 18–65 | Type 2 | 70 | 6 | Gather Health: smartphone app for patients and a smartphone app + web portal for providers. Patients uploaded self-care data and providers provided feedback. Participants could contact providers through the online portal. Patients were reminded via the app to enter self-care data. Patients also received all the care/services provided to the control condition | Usual care, with free visits, laboratory tests, transportation fees, strips and lancets | HbA1c, BMI, waist circumference, blood pressure, fasting blood glucose, lipids, medication adherence, BG testing adherence | HbA1c, BMI, fasting blood glucose, medication adherence, BG testing adherence |
RCT, randomized controlled trial; HbA1c, glycosylated haemoglobin; SMS, short message service; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index; PPBS, postprandial blood sugar; BG, blood glucose; App, application.
All studies were published between 2012 and 2017. Five studies were conducted in upper-middle-income countries (34,36,38,40,41), three in lower-middle-income countries (37,39,42) and one in a low-income country (35). Sample size ranged from 37 to 440, with a median of 100. All studies described interventions at the patient level, where information exchange took place between patients and healthcare providers. One study also incorporated a smartphone application (app) for providers that assisted the decision-making process (42). Seven out of the nine studies trialled some variation of an online portal where patients submitted diabetes-related clinical and self-care data and receive personalized recommendations based on health status (34-36,38,40-42). The other two studies trialled telephone consultations between patients and healthcare providers (37,39). Eight studies were conducted either exclusively or predominantly among people with type 2 diabetes, whilst one study (34) included people with predominantly type 1 diabetes.
The primary outcome for all studies was glycaemic control measured by at least one clinical indicator, such as HbA1c. Other clinical outcomes included fasting blood sugar, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglycerides, fructosamine, postprandial blood sugar (PPBS), fasting plasma glucose, blood pressure, weight, hip and waist circumference and body mass index (BMI). Self-reported outcomes included adherence to medication, rates of blood glucose testing, and diabetes self-care behaviours (measured using standardized instruments). Four studies did not report between-group analyses on several or all described outcome measures (35-37,39,42).
Intervention effects
Intervention effects by outcome measures
Refer to Table 2 for all between-group analyses by outcome measures. Six studies (34,36,37,40-42) compared average change in HbA1c between conditions, of which four reported greater average reduction in HbA1c levels for the intervention condition. Two studies reported P<0.001 (34,41), one reported P<0.01 (36) and one reported P=0.02 (42), while two studies (37,40) reported a non-significant difference between conditions.
Table 2
| Outcome measure | Study | Intervention | Control | Effect size, P value |
|---|---|---|---|---|
| HbA1c (mean change in %) | Moattari et al. (34) | –2.03 | –0.6 | P<0.001* |
| Zhou et al. (36) | –1.6 | –0.62 | P<0.01* | |
| Kaur et al. (37) | –0.5 | –0.17 | P=0.99 | |
| Anzaldo-Campos et al. (40) | –3.02 | –2.63 | P=0.86 | |
| Zhou et al. (41) | –1.95 | –0.79 | P<0.001* | |
| Kleinman et al. (42) | –1.5 | –0.8 | P=0.02* | |
| HbA1c (% age of patients achieving target of <7%) | Zhou et al. (36) | 66.04 | 47.27 | Significant. P value not reported* |
| Shahid et al. (39) | Not specified | Not specified | Adjusted RR =2.71; P=0.023* | |
| FBS (mean change in mg/dL) | Moattari et al. (34) | –10.87 | 1.66 | P=0.681 |
| Zhou et al. (36) | –30.1 | –12.8 | P<0.05* | |
| Kaur et al. (37) | –49.3 | –41.75 | P=0.71 | |
| Zhou et al. (41) | –34.2 | –17.3 | P<0.01* | |
| Kleinman et al. (42) | –32.6 | –23.5 | P=0.55 | |
| PPBS (mean change in mg/dL) | Kaur et al. (37) | –62.7 | –68.9 | P=0.337 |
| FPG (mean change in mmol/L) | Lee et al. (38) | 0.1 | 1.4 | P=0.112 |
| LDL (mean change in mmol/L) | Moattari et al. (34) | –0.46 | 0.28 | P<0.02* |
| Zhou et al. (36) | 0.02 | –0.26 | Not significant | |
| Lee et al. (38) | –0.1 | -0.1 | P=0.777 | |
| Anzaldo-Campos et al. (40) | –0.022 | –0.014 | Not significant | |
| Zhou et al. (41) | –0.05 | –0.08 | Not significant | |
| HDL (mean change in mmol/L) | Moattari et al. (34) | 0.31 | 0.16 | P=0.307 |
| Lee et al. (38) | 0.1 | 0.1 | P=0.887 | |
| Zhou et al. (36) | 0.04 | 0.0 | Not significant | |
| Anzaldo-Campos et al. (40) | 0.085 | 0.087 | Not significant | |
| Total cholesterol (mean change in mmol/L) | Moattari et al. (34) | 0.21 | –0.04 | P=0.69 |
| Zhou et al. (36) | –0.08 | –0.53 | Not significant | |
| Lee et al. (38) | 0.1 | 0.1 | P=0.378 | |
| Anzaldo-Campos et al. (40) | –0.76 | –0.41 | Not significant | |
| Triglycerides (mean change in mmol/L) | Moattari et al. (34) | 2.51 | –0.44 | P=0.336 |
| Zhou et al. (36) | –0.11 | –0.07 | Not significant | |
| Lee et al. (38) | 0.5 | 0.3 | P=0.421 | |
| Anzaldo-Campos et al. (40) | –2.60 | –1.66 | Not significant | |
| Fructosamine (mean change in µmol/L) | Lee et al. (38) | –19.4 | –30 | P=0.157 |
| Hypoglycemic episodes (total n) | Zhou et al. (36) | 7 | 14 | P=0.044* |
| Lee et al. (38) | 88 | 157 | OR =0.2, P=0.04* | |
| Zhou et al. (41) | 2.34 | 2.43 | Not significant | |
| Weight (mean change in kg) | Zhou et al. (41) | –0.2 | 0.2 | Not significant |
| BMI (mean change kg/m2) | Zhou et al. (36) | 0 | 0.11 | Not significant |
| Anzaldo-Campos et al. (40) | 0.23 | 0.25 | Not significant | |
| Zhou et al. (41) | –0.03 | 0.09 | Not significant | |
| Kleinman et al. (42) | –0.1 | 0.1 | P=0.53 | |
| Waist circumference (mean change in cm) | Zhou et al. (41) | 0.0 | 0.0 | Not significant |
| Hip circumference (mean change in cm) | Zhou et al. (41) | 0.0 | 0.0 | Not significant |
| SBP (mean change in mmHg) | Zhou et al. (36) | –4.02 | –2.95 | Not significant |
| Anzaldo-Campos et al. (40) | –4.05 | 0.08 | Not significant | |
| Zhou et al. (41) | –0.6 | –2 | Not significant | |
| DBP (mean change in mmHg) | Zhou et al. (36) | –2.25 | –1.84 | Not significant |
| Anzaldo-Campos et al. (40) | –3.74 | 0.14 | Not significant | |
| Zhou et al. (41) | –1.0 | –0.3 | Not significant | |
| Medication adherence (took all medication last week) | Kleinman et al. (42) | 39.0 | 12.8 | P=0.03* |
| BG testing (any last week) | Kleinman et al. (42) | 39.0 | 10.3 | P=0.01* |
| Diabetes self-care behaviours score: | ||||
| a) SDSCA | Anzaldo-Campos et al. (40) | 14.44 | 14.08 | Not significant |
| b) IMEVID | Zhou et al. (41) | 15.8 | 10.2 | P<0.01* |
*, significant at P<0.05. RR, risk ratio; OR, odds ratio. HbA1c, glycosylated haemoglobin; FBS, fasting blood sugar; PPBS, post-prandial blood sugar; FPG, fasting plasma glucose; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; BG, blood glucose; IMEVID, the Instrument to Measure Lifestyle of Type 2 Diabetes Mellitus Patients; SDSCA, Summary of Diabetes Self-Care Activities Measure.
Two studies compared the proportion of participants achieving target HbA1c levels between conditions (36,39). Zhou et al. (36) reported that 66.04% of the intervention group, compared to 42.27% of the control group achieved target HbA1c levels (i.e., HbA1c <7.0%) post-intervention, a difference they reported to be statistically significant, however the p-value was not reported. The other study by Shahid et al. (39) did not report the proportion in each group that achieved normal HbA1c levels, however reported the adjusted risk ratio =2.71 (P=0.023).
Out of the five studies that analysed change in fasting blood sugar, two studies reported significant intervention effects (36,41). In these two studies, the intervention groups reported average reductions of 30.1 and 34.2 mg/dL, compared to the average reduction among the control groups—12.8 and 17.3 mg/dL, respectively (36,41). The study by Zhou et al. (41) reported P<0.01, and the study by Zhou et al. (36) reported P<0.05.
Out of the five studies that analysed change in LDL, one study reported significantly greater improvement among the intervention group (reduction of 0.46 mmol/L) compared to control (increase of 0.28 mmol/L), with P<0.02 (34). Four studies reported no effect of the intervention on LDL (37,38,40,41). None of the included studies reported statistically significant intervention effects on any other clinical outcome measure (i.e., HDL, triglycerides, fructosamine, PPBS, fasting plasma glucose, total cholesterol, weight, BMI, waist and hip circumference, systolic and diastolic blood pressure).
Out of three studies that compared the number of self-reported hypoglycemic episodes between groups, two reported that the intervention groups experienced significantly fewer than the comparison groups: 88 vs. 157 episodes, with P<0.04 (38); and 7 vs. 14 episodes, with P<0.05 (36), whilst the third reported no difference (41).
The single study that tested the effect of the intervention on self-reported medication adherence, reported that a significantly greater number of people from the intervention group improved in taking all prescribed medication, compared to the control group (P=0.03) (42). The same study reported that the positive change in number of people testing blood glucose post-intervention was significantly stronger among the intervention group, compared to control (P=0.01) (42).
Two studies compared the control and intervention groups on diabetes self-care behaviours over the course of the study using standardized multi-item scales (40,41). The study by Zhou et al. (41) reported significantly greater improvements on the Instrument to Measure Lifestyle of Type 2 Diabetes Mellitus Patients (IMEVID) among intervention participants (P<0.01), whilst the study by Anzaldo-Campos et al. (40) reported no between-group difference on the Summary of Diabetes Self-Care Activities Measure (SDSCA).
Intervention effects by intervention type
Five out of the seven studies that trialled variations of a mobile portal for transmitting information between patients and healthcare providers reported significant positive intervention effects on at least one measure of glycaemic control. Of these, four studies reported greater average reductions in HbA1c levels for the intervention group (34,36,41,42). The fifth study reported significantly fewer hypoglycemic episodes among the intervention group, compared to control (38), an effect also reported in the study by Zhou et al. (36). Other positive intervention effects included greater average reduction in fasting blood sugar levels (36), larger proportion of people achieving target HbA1c levels (36), and significantly better LDL change scores (34). Of these studies, three evaluated the intervention on diabetes self-care behaviours, with two observing positive intervention effects (41,42). Kleinman et al. reported significant intervention effects on medication adherence and blood glucose testing (42). Two studies administered multi-item measures to derive a total diabetes self-care score. Of these, Zhou et al. (41) observed significantly greater average improvements for the intervention group, compared to control, whereas Anzaldo-Campos et al. (40) reported no difference between groups. The studies by Anzaldo-Campos et al. (40) and Takenga et al. (35) did not observe positive intervention effects on any outcome measure, potentially due in part to the absence of between-group analyses on some or all outcome measures.
Of the two studies that trialled telephone consultations between patients and healthcare providers, the study by Shahid et al. (39) reported a significantly greater proportion of people achieving normal HbA1c levels in the intervention group, compared to control with an adjusted risk ratio =2.71 (P=0.023). The study by Kaur et al. (37) reported no effect of the intervention on any diabetes outcome measure.
Summary
In summary, six out of nine included studies reported significant, positive effects of the intervention on at least one clinical or self-reported measure of glycaemic control (34,36,38,39,41,42). The mHealth intervention was associated with greater reductions in HbA1c in four studies (34,36,41,42), fewer episodes of hypoglycemia in two studies (36,38), improved fasting blood sugar in one study (36), greater proportions of people achieving target HbA1c in two studies (36,39), improved LDL in one study (34), improvements in both medication adherence and blood glucose testing in one study (42), and improvements in overall self-care behaviour in one study (41).
Included studies were highly heterogeneous due to important differences in populations, interventions, study designs, outcomes and results. Based on this assessment, a meta-analysis was not undertaken.
Discussion
This review of published evidence for the effectiveness of mHealth interventions for diabetes care in LMICs, found that most of the included studies provide some evidence of a positive intervention effect on clinical diabetes-related outcomes. These results are somewhat consistent with the results of previous reviews, which have largely reported positive intervention effects (14,21,24,26,27). Whilst only three studies reviewed here investigated the effect of the mHealth intervention on key diabetes-related behavioural outcomes, two studies (41,42) reported positive intervention effects which were consistent with previous reviews (14,27).
In the current review, most studies where patients and healthcare providers exchanged information via an online portal, reported the intervention to be associated with greater improvement in blood glucose outcomes. This is consistent with a former review of this type of mHealth intervention in HICs, which reported positive intervention effects on pooled HbA1c (43). Thus, these types of mHealth interventions show promise in both low- and high-resource settings.
This review reported mixed evidence for an effect of telephone consultations between healthcare providers and patients on clinical diabetes outcomes. Previous reviews (9,24) reported that telephone consultations were associated with improved HbA1c levels, a conclusion only partially supported here. One previous review (9) found evidence supporting an effect of telephone consultations on diabetes self-care behaviours, whilst another reported no effect (24). Neither of the two studies that evaluated telephone consultations in this review reported testing behavioural outcomes, which impeded conclusions about such outcomes.
The present review demonstrates promising, albeit limited evidence for the effectiveness of mHealth interventions on glycaemic control in LMICs. Furthermore, the review process identified several additional studies of mHealth applications for diabetes care in LMICs which were excluded because of study design, failure to report on any of the outcome measures, or were research protocols (44-46). Thus, there is evidence of additional mHealth interventions for diabetes care in LMICs other than those reviewed here. In addition, a survey conducted by the World Health Organization indicated that as few as 12% of mHealth initiatives are evaluated (47). Therefore, there is great opportunity to add to the evidence base by conducting evaluations of existing and proposed mHealth applications using rigorous study designs and assessing effectiveness on key diabetes outcome measures, both clinical and behavioural.
Several limitations of this review warrant consideration. Firstly, all included studies suffered from multiple methodological or reporting weaknesses. A detailed analysis of included studies’ performance on each domain of the Cochrane Collaboration’s risk of bias assessment tool is summarised in Table 3. Overall, the brevity with which study methods were described made it difficult to assess all the studies on at least two domains of the tool. Randomization procedures, methods for concealing allocation procedures and processes for handling missing data/participant dropout in analyses were generally poorly described. While most studies reported the baseline characteristics for each group separately, three studies did not establish group equivalence at baseline (34,35,38). Several studies (35,38) also failed to report testing some or all outcome measures as between-group comparisons, which was contradictory to their study design.
Table 3
| Domain | Moattari et al. (34) | Takenga et al. (35) | Zhou et al. (36) | Kaur et al. (37) | Lee et al. (38) | Shahid et al. (39) | Anzaldo-Campos et al. (40) | Zhou et al. (41) | Kleinman et al. (42) |
|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | √ | ? | √ | √ | ? | X | ? | ? | √ |
| Allocation concealment | ? | ? | ? | ? | ? | ? | ? | ? | √ |
| Blinding of participants and personnel | ? | ? | ? | ? | ? | X | ? | ? | ? |
| Blinding of outcome assessment | √ | X | ? | ? | ? | ? | ? | ? | √ |
| Incomplete outcome data | √ | X | X | √ | ? | √ | √ | ? | ? |
| Selective reporting | X | X | √ | X | X | X | √ | ? | X |
√, low risk; X, high risk; ?, unclear risk.
This review has established that further studies in this field are needed. Future studies should deliver standard and equivalent care to all study participants apart from the trialled mHealth intervention. Additionally, studies should conduct and report the results of appropriate between-group analyses on all measured outcomes, including at baseline, to enable a comprehensive assessment of the intervention’s effectiveness as well as adverse effects.
Additionally, given the chronicity of diabetes, future studies should also evaluate mHealth interventions over longer treatment and follow-up periods. There is widespread recognition that patients face many barriers to making behavioural changes to improve diabetes outcomes (48). Greater exposure to the intervention may give participants more opportunity to understand, adapt to and integrate the new program into their lives.
This review reported on just one study that included a sample of people with mostly type 1 diabetes (34). This review found preliminary evidence that the effects of mHealth interventions may differ according to type of diabetes. Thus, future studies should examine the effects of mHealth interventions on people with type 1 as well as type 2 diabetes either in separate studies or reporting on the outcomes separately to allow for comparison.
In addition, analyzing the effect of the intervention on both behavioural diabetes self-care practices (e.g., diet, exercise, blood glucose monitoring and medication adherence) and clinical outcome measures within the one study is recommended to determine whether intervention effects on glycaemic control occur irrespective of impact on behaviour change. Furthermore, evaluating both types of outcomes over longer periods may elucidate change trajectories, for instance, behaviour change preceding changes in clinical markers. In addition, given that a key justification for developing mHealth interventions is their cost-saving potential, future studies should assess cost effectiveness.
Conclusions
This review synthesized the current evidence for the effectiveness of mHealth interventions for diabetes management in LMICs and highlighted numerous research gaps and methodological challenges in the existing research. The findings demonstrate that there is promising, albeit limited evidence that mHealth interventions in LMICs can have positive effects on glycaemic control and self-care behaviours. However, the field of mHealth for diabetes management in LMICs is still in its infancy, and there is a dearth of experimental studies adequately evaluating these interventions on key clinical or behavioural outcomes. This highlights the need for more rigorous evaluation of these interventions to provide a stronger research base for policy makers and clinicians.
Acknowledgments
Sincere thanks go to Nicole Butcher for providing detailed editing of the document in preparation for submission.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp.2018.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Venkataraman K, Kannan A, Mohan V. Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Ctries 2009;29:103-9. [Crossref] [PubMed]
- Gakidou E, Mallinger L, Abbott-Klafter J, et al. Management of diabetes and associated cardiovascular risk factors in seven countries: A comparison of data from national health examination surveys. Bull World Health Organ 2011;89:172-83. [Crossref] [PubMed]
- Colagiuri R, Brown J, Dain K. Global Diabetes Plan 2011-2021. Brussels, Belgium: International Diabetes Federation, 2011.
- Zimmet PZ, Magliano DJ, Herman WH, et al. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol 2014;2:56-64. [Crossref] [PubMed]
- Suksomboon N, Poolsup N, Nge YL. Impact of phone call intervention on glycemic control in diabetes patients: A systematic review and meta-analysis of randomized, controlled trials. PloS One 2014;9:e89207 [Crossref] [PubMed]
- Smith R, Exeter C. Countering non-communicable diseases through innovation: Report of the Non-Communicable Disease Working Group 2012. The Global Health Policy Summit; Imperial College, London, 2012.
- Harrison S, Stadler M, Ismail K, et al. Are patients with diabetes mellitus satisfied with technologies used to assist with diabetes management and coping?: A structured review. Diabetes Technol Ther 2014;16:771-83. [Crossref] [PubMed]
- Faridi Z, Liberti L, Shuval K, et al. Evaluating the impact of mobile telephone technology on type 2 diabetic patients’ self‐management: The NICHE pilot study. J Eval Clin Pract 2008;14:465-9. [Crossref] [PubMed]
- Cassimatis M, Kavanagh DJ. Effects of type 2 diabetes behavioural telehealth interventions on glycaemic control and adherence: A systematic review. J Telemed Telecare 2012;18:447-50. [Crossref] [PubMed]
- International Diabetes Federation Clinical Guidelines Taskforce. Global guidelines for Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation, 2012.
- CARRS Trial Writing Group. Improving diabetes care: Multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia—The CARRS Multi-center Translation Trial. Diabetes Res Clin Pract 2012;98:285-94. [Crossref] [PubMed]
- Ramachandran A, Shetty AS, Nandhitha A, et al. Type 2 diabetes in India: Challenges and possible solutions. Available online: http://www.apiindia.org/medicine_update_2013/chap40.pdf
- Kalra S, Unnikrishnan AG, Skovlund SE. Patient empowerment in endocrinology. Indian J Endocrinol Metab 2012;16:1-3. [Crossref] [PubMed]
- Russell-Minda E, Jutai J, Speechley M, et al. Health technologies for monitoring and managing diabetes: A systematic review. J Diabetes Sci Technol 2009;3:1460-71. [Crossref] [PubMed]
- Jarvis J, Skinner T, Carey M, et al. How can structured self‐management patient education improve outcomes in people with type 2 diabetes? Diabetes Obes Metab 2010;12:12-9. [Crossref] [PubMed]
- Carter EL, Nunlee-Bland G, Callender C. A patient-centric provider-assisted diabetes telehealth self-management intervention for urban minorities. Perspect Health Inf Manag 2011;8:1b. [PubMed]
- Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diabetes Care 2009;32:S87-S94. [Crossref] [PubMed]
- Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health 2014;14:188. [Crossref] [PubMed]
- World Health Organization. Telemedicine: Opportunities and developments in member states. Report on the second global survey on eHealth. Geneva: World Health Organization, 2010.
- Martinez PR. A qualitative study on patient perceptions towards mHealth technology among high risk, chronic disease patients. Doctoral dissertation; Harvard Medical School, 2015.
- Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med 2013;10:e1001362 [Crossref] [PubMed]
- Chib A, van Velthoven MH, Car J. mHealth adoption in low-resource environments: A review of the use of mobile healthcare in developing countries. J Health Commun 2015;20:4-34. [Crossref] [PubMed]
- Beratarrechea A, Lee AG, Willner JM, et al. The impact of mobile health interventions on chronic disease outcomes in developing countries: A systematic review. Telemed J E Health 2014;20:75-82. [Crossref] [PubMed]
- Wu L, Forbes A, Griffiths P, et al. Telephone follow‐up to improve glycaemic control in patients with Type 2 diabetes: Systematic review and meta‐analysis of controlled trials. Diabet Med 2010;27:1217-25. [Crossref] [PubMed]
- Hawkins SY. Improving glycemic control in older adults using a videophone motivational diabetes self-management intervention. Res Theory Nurs Pract 2010;24:217-32. [Crossref] [PubMed]
- Tao D, Or CK. Effects of self-management health information technology on glycaemic control for patients with diabetes: A meta-analysis of randomized controlled trials. J Telemed Telecare 2013;19:133-43. [Crossref] [PubMed]
- Krishna S, Boren SA. Diabetes self-management care via cell phone: A systematic review. J Diabetes Sci Technol 2008;2:509-17. [Crossref] [PubMed]
- Holtz B, Lauckner C. Diabetes management via mobile phones: A systematic review. Telemed J E Health 2012;18:175-84. [Crossref] [PubMed]
- Graziano JA, Gross CR. The effects of isolated telephone interventions on glycemic control in type 2 diabetes: A literature review. ANS Adv Nurs Sci 2009;32:E28-E41. [Crossref] [PubMed]
- Braun R, Catalani C, Wimbush J, et al. Community health workers and mobile technology: A systematic review of the literature. PloS One 2013;8:e65772 [Crossref] [PubMed]
- Free C, Phillips G, Felix L, et al. The effectiveness of M-health technologies for improving health and health services: A systematic review protocol. BMC Res Notes 2010;3:250. [Crossref] [PubMed]
- Felipe J, Abdon A, Kumar U. Tracking the middle-income trap: What is it, who is in it, and why? Working Paper. Levy Economics Institute, Bard College; NY, 2012.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Moattari M, Hashemi M, Dabbaghmanesh MH. The impact of electronic education on metabolic control indicators in patients with diabetes who need insulin: A randomised clinical control trial. J Clin Nurs 2013;22:32-8. [Crossref] [PubMed]
- Takenga C, Berndt RD, Musongya O, et al. An ICT-based diabetes management system tested for health care delivery in the african context. Int J Telemed Appl 2014;2014:437307 [Crossref] [PubMed]
- Zhou P, Xu L, Liu X, et al. Web-based telemedicine for management of type 2 diabetes through glucose uploads: A randomized controlled trial. Int J Clin Exp Pathol 2014;7:8848-54. [PubMed]
- Kaur R, Kajal KS, Kaur A, et al. Telephonic consultation and follow-up in diabetics: Impact on metabolic profile, quality of life, and patient compliance. N Am J Med Sci 2015;7:199-207. [Crossref] [PubMed]
- Lee JY, Lee SW, Nasir NH, et al. Diabetes telemonitoring reduces the risk of hypoglycaemia during Ramadan: A pilot randomized controlled study. Diabet Med 2015;32:1658-61. [Crossref] [PubMed]
- Shahid M, Mahar SA, Shaikh S, et al. Mobile phone intervention to improve diabetes care in rural areas of Pakistan: A randomized controlled trial. J Coll Physicians Surg Pak 2015;25:166-71. [PubMed]
- Anzaldo-Campos MC, Contreas S, Vargas-Ojeda A, et al. Dulce Wireless Tijuana: A randomized control trial evaluating the impact of Project Dulce and short-term mobile technology on glycemic control in a family medicine clinic in Northern Mexico. Diabetes Technol Ther 2016;18:240-51. [Crossref] [PubMed]
- Zhou W, Chen M, Yuan J, et al. Welltang. A smart phone-based diabetes management application – Improves blood glucose control in Chinese people with diabetes. Diabetes Res Clin Pract 2016;116:105-10. [Crossref] [PubMed]
- Kleinman NJ, Shah A, Shah S, et al. Improved medication adherence and frequency of blood glucose self-testing using an m-Health platform versus usual care in a multisite randomized clinical trial among people with Type 2 Diabetes in India. Telemed J E Health 2017;23:733-40. [PubMed]
- Huang Z, Tao H, Meng Q, et al. Management of endocrine disease: Effects of telecare intervention on glycemic control in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur J Endocrinol. 2015;172:R93-R101. [Crossref] [PubMed]
- Islam SM, Lechner A, Ferrari U, et al. Mobile phone intervention for increasing adherence to treatment for type 2 diabetes in an urban area of Bangladesh: Protocol for a randomized controlled trial. BMC Health Serv Res 2014;14:586. [Crossref] [PubMed]
- Kim YJ, Rhee SY, Byun JK, et al. A smartphone application significantly improved diabetes self-care activities with high user satisfaction. Diabetes Metab J 2015;39:207-17. [Crossref] [PubMed]
- Hopewell S, Loudon K, Clarke MJ, et al. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;MR000006 [PubMed]
- World Health Organization. mHealth: New horizons in health through mobile technologies: Second global survey on ehealth. Geneva, Switzerland: World Health Organization, 2011.
- Asaad G, Soria-Contreras DC, Bell RC, et al. Effectiveness of a lifestyle intervention in patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial. Healthcare (Basel) 2016;4: [Crossref] [PubMed]
Cite this article as: Johnston L, Zemanek J, Reeve MJ, Grills N. The evidence for using mHealth technologies for diabetes management in low- and middle-income countries. J Hosp Manag Health Policy 2018;2:35.