Sarcopenia in non-small cell lung cancer patients underwent pulmonary surgery: clinical outcome and cost effectiveness
Introduction
Lung cancer is the leading cause of cancer death in several countries, in particular non-small cell lung cancer (NSCLC) accounts for about 75–80% of primary lung cancer (1,2). Surgical resection is a potentially curative treatment for NSCLC, but recurrence could develop in 27–38% of patients after surgical resection of stage I NSCLC (3). Tumor-specific factors, such as tumor size, node, and metastasis (TNM staging system) are important to determinate the postoperative prognosis (4). However, physical factors, such as body mass index and sarcopenia, are also important for postoperative prognosis (5-7). Sarcopenia is a physical component of cachexia syndrome, characterized by significant loss of skeletal muscle mass and function. Furthermore, it is associated with poor quality of life, a risk of adverse outcomes, such as physical disability, and death (8).
For this reasons, sarcopenia is identified as a prognostic factor for various cancers, especially lung and colo-rectal cancer (9-11). In fact, a reduction of the skeletal muscle mass can be reflected on a loss of respiratory muscle mass and strength. The importance of this condition is more relevant when the diaphragm muscle is affected. Determination of sarcopenia can be achieved using various imaging techniques to assess skeletal muscle mass, such as computed tomography (CT), dual X-ray absorptiometry (DEXA), magnetic resonance imaging (MRI) and bio-impedance analysis (BIA) (8,12,13). In particular, CT scan has become a routine diagnostic tool for lung cancer staging and some authors have evaluated the depletion of muscle mass in various cancers, using this radiological technique (9-11). Measuring psoas muscle index (PMI) at the third lumbar level on CT was proposed for assessing the skeletal muscle mass (13). It is a convenient and inexpensive method that could be used in routine practice.
People with an active cancer have a reduced amount of lean body mass while fat mass is stable or increased (sarcopenic obesity) (9). Sarcopenia has been correlated with NSCLC by different studies, which has shown a reduction of the global survivor rate of sarcopenic patients who undergo thoracic surgery (14).
We conducted a retrospective study to investigate the link between sarcopenia and postoperative outcomes of surgical resected lung cancer patients, using preoperative CT for lung cancer staging. We also took in consideration the impact of sarcopenia on the national health cost and five years overall survival rate.
Methods
Patients affected by NSCLC, stage I–IIIa, undergoing radical surgery, including lobectomy and bi-lobectomy, with a mediastinal lymphadenectomy, were enrolled. All surgical procedures were performed between 2002 and 2012 at University Campus Bio-Medico of Rome (UCBM). We excluded other types of cancer or synchronous cancer with a non-pulmonary primitivity, metastatic disease, stage IV or patients with a severe obesity (BMI >30 kg/m2).
Forty-two patients were considered suitable for the study, according to the inclusion and exclusion criteria. Among them, 28 were male and 14 females with average age of 72 years.
We performed the surgical procedures with the patient under general anaesthesia with lateral thoracotomy access (open surgery). We inserted two drainages into the pleural cavity, apical drainage for any air leaks, basal drainage for the fluid leaks.
The Ethics Committee of Campus Bio-Medico University of Rome approved this study (No: 882017).
Measurements
The identification of Sarcopenia status was reached with the use of CT, with two slices at the level of the third lumbar level (L3), allowing the measure of the cross-sectional area from L3 to the iliac crest, expressed in cm2. The anatomic section includes the following muscles: psoas, para-vertebral, intercostal, oblique, transverses and rectus. Using Software Osirix and Hounsfield Units’s window, variables among patients were between −5–150 HU on average (range: −40/40–150 HU).
We identified sarcopenic and non-sarcopenic patients, using the Sarcopenia Index, obtained from dividing the area find with the CT scan measuring, by the patient’s height (cm2/m2), 52.4 cm2/m2 for the man and 38.5 cm2/m2 for the woman.
We collected clinical parameters, including age, sex, weight (kg), height (cm), and BMI (kg/m2), glycemia (mg/dL) and arterial blood gases (ABG). The last two parameters were measured in two different conditions: pre operation, in the first and second postoperative day.
Finally, the evaluation of the infective risk and the health expenditure included the analysis of days of hospitalizations, duration of permanence of thoracic drainages, both apical and basal, expressed in days.
The result was considered statistically significant at the significance level of P<0.05. Survival curves were constructed using Kaplan-Meier method and overall survival rates were compared using log-rank test.
Results
Of the 42 patients suitable for the study, 13 were sarcopenic (31%) and 29 were non-sarcopenic (69%). Of the 13-sarcopenic patient, 9 were male and 4 females. We collected the median value of the parameters. BMI is 24.4 kg/m2 on sarcopenic patients and 27.3 kg/m2 on non-sarcopenic patients. Days of hospitalizations are 10 days in sarcopenic patients and 8.5 days in non-sarcopenic patients. Duration of permanence of the thoracic drainage is 7 days in sarcopenic patients and 5 days in non-sarcopenic patients.
The AGB in the first postoperative day showed: pO2 =74.4 mmHg, pCO2 =40.25 mmHg, SO2 =95.75% in sarcopenic patients and pO2 =87.3 mmHg, pCO2 =40 mmHg, SO2 =97.1% in non-sarcopenic patients. The AGB in the second postoperative day showed: pO2 =72.9 mmHg, pCO2 =36.9 mmHg, SO2 =96.3% in sarcopenic patients and pO2 =79.4 mmHg, pCO2 =37.6 mmHg, SO2 =97.6% in non-sarcopenic patients.
Preoperative glycemia is 105 mg/dL in sarcopenic patients and 101 mg/dL in non-sarcopenic patients while postoperative glycemia is 132 mg/dL in sarcopenic patients and 113 mg/dL in non-sarcopenic patients (Table 1).
Table 1
| Variables | Sarcopenic | Non sarcopenic |
|---|---|---|
| Number of patients (%) | 13 (31%) | 29 (69%) |
| Age (median) | 77 | 69 |
| Sex | ||
| Male (%) | 9 (32%) | 19 (68%) |
| Female (%) | 4 (29%) | 10 (71%) |
| BMI (kg/m2) | 24.4 | 27.3 |
| Days of hospitalizations (dd) (median) | 10 | 8.5 |
| Permanence of apical drainage (dd) (median) | 4 | 4 |
| Permanence of basal drainage (dd) (median) | 7 | 5 |
| AGB I postoperative day | ||
| pO2 (mmHg) (median) | 74.4 | 87.3 |
| pCO2 (mmHg) (median) | 40.25 | 40 |
| SO2 (%) (median) | 95.75 | 97.1 |
| AGB II postoperative day | ||
| pO2 (mmHg) (median) | 72.9 | 79.4 |
| pCO2 (mmHg) (median) | 36.9 | 37.6 |
| SO2 (%) (median) | 96.3 | 97.6 |
| Glycemia (mg/dL) | ||
| Preoperative (median) | 105 | 101 |
| Postoperative (median) | 132 | 113 |
Regarding the costs and the established reimbursement given to the UCBM between 2002–2012, on average, the care of a patient with NSCLC who underwent surgery for lobectomy and lymphadenectomy, amounted to 4,187 euro for non sarcopenic patients while, the same procedure, for a sarcopenic patient is about 5,686.75 euro (difference of 1,499.75 euro). This gap is due to 1.5 days of hospitalization more of non-sarcopenic patients than non-sarcopenic ones (10 vs. 8.5 days). We obtained the increase in health care spending, multiplying this value, 1.5, by the following costs: bed place (842 euro per day), geriatric counselling (65 euro each counselling), and blood tests (34 euro per day). The national health system refunded 8,067 euro for lung lobectomy hospitalization, so the UCBM received 3,880 euro (euro 8,067−4,187) for each non sarcopenic patient, while, because of the higher cost for the care, they received 2,380.25 euro (euro 8,067−5,686.75) for each sarcopenic patient (Tables 2-4).
Table 2
| Cost type | Cost detail | Value (€) |
|---|---|---|
| Proceeds from discharge | Proceeds from DRG | 8,067.00 |
| Cost | 4,187.42 | |
| Acceptance | Per discharge | 9.40 |
| Medical records | Per discharge | 23.10 |
| Meals | Per day of hospitalization (no ICU) | 80.50 |
| Prosthetic materials | Total | 23.58 |
| Materials nursing department | 0.78 | |
| Materials operating room | 22.80 | |
| Drugs | All | 538.53 |
| Nursing department | 75.57 | |
| Health care | Total | 628.27 |
| Analysis laboratory | 243.38 | |
| Pathological anatomy | 155.10 | |
| Radiology | 77.45 | |
| Anaesthesia and pain therapy | 13.63 | |
| Cardiology | 32.28 | |
| Haematology | 13.63 | |
| Geriatrics | 64.56 | |
| Lab-haematology | 28.24 | |
| Other materials | 233.3 | |
| Garrisons | 1,563 | |
| Chancellery | 4.14 | |
| Laboratory materials | 35.3 | |
| Haemocomponents | Haemocomponents | 136.33 |
| Waste disposal | Per hospitalization | 205.00 |
| Laundry | Per day of hospitalization (no ICU) | 138.00 |
| Bed place | Per day of hospitalization SSN (no ICU) | 704.00 |
Table 3
| Health care | Additional health costs for sarcopenic patients |
|---|---|
| Meals | (Euro) 80.5×1.5 (days) =120.75 euro |
| Bed place | (Euro) 842×1.5 (days) =1,263 euro |
| Analysis laboratory | (Euro) 34×1.5 (days) =51 euro |
| Geriatrics counselling | 65 euro |
| Total ×1.5 days | 1,499.75 euro |
Table 4
| Variable | Outcome |
|---|---|
| DRG 75-National Health System Refund | 8,067 euro |
| UCBM cost for non-sarcopenic patient | 4,187 euro |
| UCBM proceeds for non-sarcopenic patient | 8,067−4,187=3,380 euro |
| UCBM cost for sarcopenic patient | 4,187+1,499.75=5,686.75 euro |
| UCBM proceeds for sarcopenic patient | 8,067−5,686.75=2,380.25 euro |
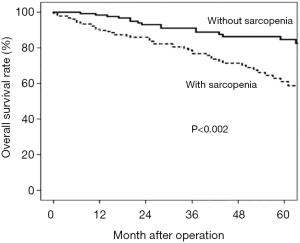
The five-year survival rate was 57% in patients with sarcopenia and 88% in those without. Multivariate analysis revealed that sarcopenia was an independent unfavourable prognostic factor (P<0.002) (Figure 1).
Discussion
Basing on GLISTEN Study (15), there is a high prevalence of sarcopenia in the Italian population.
In our study, we focused on the role of sarcopenia in patients with NSCLC as an influential factor for the patient’s outcome.
First of all, the healthcare costs are higher among sarcopenic patients (statistically significant: P<0.0037).
Concerning the permanence of thoracic drainages, the apical drainage has the same permanence (4 days) in both sarcopenic and non-sarcopenic patients, while the basal drainage has a longer permanence in sarcopenic patients (7 vs. 5 days; statistically significant: P<0.0043).
The longer permanence of the basal drainage could lead to infections: one of the major complications related to the drainage. Previous studies demonstrated that another condition could increase the risk of pulmonary complication and infections: the aging-related weakness of the diaphragm muscle due to atrophy of fast muscle fibers (tipe IIx and/or IIb) (16). Health expenditure could be burdened by the development of an infective complication and the consequent necessity of counselling, tests, and prolonged hospitalization. On the other hand, both conditions can exasperate patients’ outcome. Then we analysed the respiratory parameters (pO2, pCO2 and SO2) in the first and second postoperative days. The values found are similar in the two groups, but sarcopenic patients showed a slower recovery of the normal range (not statistically significant: P<0.21). This agrees with the negative effect of sarcopenia on the lung function and ventilation mechanism.
Al last, the glycaemic median (mg/dL) is higher in sarcopenic patients than in non sarcopenic, both in preoperative (105 vs. 101) and postoperative (132 vs. 113), favouring a condition of insulin-resistance in sarcopenic patients (statistically significant: preoperative P<0.0002; postoperative P<0.0001). In these patients, it takes place a down-regulation of anabolic pathways such as IGF-1 and androgen (15,16). The high glycaemic level induced the loss of muscle mass and the development of sarcopenia. Different mechanisms act in this process, and, most of them, are due to the inflammation (17,18). People with metabolic syndrome, insulin-resistance, or type 2 diabetes have an increased level of adipokines (TNFa, IL6), inflammatory cytokines correlated with muscle mass loss. Resistin levels are increased too, which has an anti-myogenic effect activating the NfkB pathway and altering the metabolism of the muscle (18). Local inflammation reprograms the skeletal muscle transcriptome leading to altered expression of pro-inflammatory and pro-fibrotic genes (19).
High blood content of TNF-a, during cachexia, increases muscle cell apoptosis; AGEs change the bones, muscles and vessels structure because of the production of IL-6.
Other studies demonstrated the association between sarcopenia, malnutrition and inflammation markers. A harmful diet, with a high intake of fatty meals, can even affect our mitochondria.
On one side, it has been demonstrated what the positive effect of supplementation of the diet with protein, calories, amino-acids, and vitamin D as well as physical exercise can reduce on the loss of muscle mass (20). The level of inflammatory marker (IL6 and TNFα) is inversely proportional with the albumin level, so a normalization of the protein level, especially in the post-operative, could reduce inflammation (21).
On the other side, some studies provided the beneficial impact of AIDs (non-selective Cox and selective Cox-2 inhibitors) on performance and muscle weakness, reducing the grade inflammation. For example: piroxicam decreases the baseline values in IL-6, while celecoxib showed a significant decrease in IL-10 (17). As different studies underline, pro-inflammatory cytokine IL-6 has a rule in cachexia and cancer growth, up to consider the target therapy against IL-6 as anti-tumour and useful for the treatment of the muscle loss (17-21).
In conclusion, our study demonstrates the impact of sarcopenia in different ambits, from the clinical outcomes to the health expenditure. Sarcopenia, the “big geriatric syndrome”, lead with her the development of a set of comorbidities that makes the care of these patients difficult.
A possible treatment to prevent and relieve the effect of sarcopenia can be physical exercise, diet and anti-inflammatory drugs.
Preventing the insurgence of sarcopenia is therefore essential, creating protocol of physical activity, diet and therapy aimed at reducing the loss of strength and muscle mass that physiologically decrease with the age. This is fundamental for the patients, to improve the postoperative outcomes, and for the Health System, to reduce healthcare-related costs.
Naturally, these data need to be confirmed in a more powerful study, and the economic impact of these patients recorded outcomes should be investigated as well.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp.2019.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. The Ethics Committee of Campus Bio-Medico University of Rome approved this study (No: 882017).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheng TY, Cramb SM, Baade PD, et al. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor. Characteristics. J Thorac Oncol 2016;11:1653-71. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the Diagnosis of Lung Cancer Establishing the Diagnosis of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e142S-e165S.
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Detterbeck FC, Franklin WA, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: background data and proposals for the classification of lung cancer with separate tumor nodules in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:651-5.
- Sepesi B, Gold K, Correa A, et al. The Influence of Body Mass Index on Overall Survival Following Surgical Resection of Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1280-7. [Crossref] [PubMed]
- Nagata M, Ito H, Matsuzaki T, et al. Body mass index, C-reactive protein and survival in smokers undergoing lobectomy for lung cancer. Eur J Cardiothorac Surg 2017;51:1164-70. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: A comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in 284 Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following 292 resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. [Crossref] [PubMed]
- Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015;157:1088-98. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5. [Crossref] [PubMed]
- Nakamura R, Inage Y, Tobita R, et al. Sarcopenia in Resected NSCLC: Effect on Postoperative Outcomes. J Thorac Oncol 2018;13:895-903. [Crossref] [PubMed]
- Bianchi L, Abete P, Bellelli GGLISTEN Group Investigators, et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J Gerontol A Biol Sci Med Sci 2017;72:1575-81. [Crossref] [PubMed]
- Ferrari R, Caram LM, Faganello MM, et al. Relation between systemic inflammatory markers, peripheral muscle mass, and strength in limb muscles in stable COPD patients. Int J Chron Obstruct Pulmon Dis 2015;10:1553-8. [Crossref] [PubMed]
- Kamijo Y, Kanda E, Ishibashi Y, et al. Sarcopenia and frailty in pd: impact on mortality, malnutrition, and inflammation. Perit Dial Int 2018;38:447-54. [Crossref] [PubMed]
- O'Leary MF, Wallace GR, Davis ET, et al. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin-mediated activation of NfkB. Sci Rep 2018;8:15360. [Crossref] [PubMed]
- Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia Exacerbates Obesity-Associated Insulin Resistance and Dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS One 2010;5:e10805 [Crossref] [PubMed]
- Okugawa Y, Toiyama Y, Yamamoto A, et al. Close Relationship Between Immunological/Inflammatory Markers and Myopenia and Myosteatosis in Patients with Colorectal Cancer: A Propensity Score Matching Analysis. JPEN J Parenter Enteral Nutr 2019;43:508-15. [Crossref] [PubMed]
- Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016;24:2075-84. [Crossref] [PubMed]
Cite this article as: Frasca L, Carannante F, Longo F, Marziali V, Crucitti P. Sarcopenia in non-small cell lung cancer patients underwent pulmonary surgery: clinical outcome and cost effectiveness. J Hosp Manag Health Policy 2019;3:25.